Abstract
White-coat hypertension (WCH), commonly found in pseudoresistant hypertension, does not pose higher cardiovascular risk than hypertensive status. However, when the decrease of the out-of-office blood pressure does not reach normal levels – the white-coat effect (WCE) – the repercussions are still obscure. We investigated the repercussions of the WCE in myocardial perfusion in resistant hypertension (RHTN). We enrolled 129 asymptomatic RHTN subjects – divided into WCE (n = 63) and non-WCE (n = 66) – to perform rest and stress myocardial perfusion scintigraphy and biochemical tests. Groups were equal regarding age, gender and body mass index. There was a high prevalence of WCE (49%). WCE was associated with higher prevalence of myocardial ischemia (49.2% vs 7.6%, p < 0.001), microalbuminuria (60.3% vs 36.4%, p = 0.01) and higher heart rate (72 [64–80] vs 64 [60–69], p < 0.001), compared with non-WCE patients. On an adjusted logistic regression, heart rate was considered a predictor of WCE (OR = 1.10, 95% CI 1.04–1.15; p < 0.001), but not MA (OR = 1.8, 95% CI 0.8–3.9; p = 0.15). On a second model of adjusted logistic regression, WCE was an independent predictor of myocardial ischemia (OR = 14.7, 95% CI 4.8–44.8; p < 0.001). We found a high prevalence of WCE in RHTN, and this effect may predict silent myocardial ischemia in this subset of hypertensive patients. In this group of hypertensives special attention should be given to the WCE.
Introduction
Cardiovascular disease (CVD) is still the major cause of overall mortality in most of Western countries. Coronary heart disease (CHD) is responsible for 16% of all deaths, and causes one heart attack about every 34 s and one death every minute, in the USA alone (Citation1). Hypertension is a major risk factor for CVD, where an increase of 10 mmHg can almost triple the relative risk of CHD (Citation2). However, only 53% of the known hypertensive subjects are at their blood pressure (BP) goal (Citation1). A small but significant number of these uncontrolled patients (varying from 8.9% to 14.5%) (Citation3–5) are considered to have resistant hypertension (RHTN) (Citation6), presenting worse cardiovascular outcomes (Citation7).
The white-coat effect (WCE – a phenomenon where the patient has a lower out-of-office BP than that measured in the clinic) poses a high prevalence in RHTN (20-52%) (Citation8,Citation9). Its occurrence has been attributed to mental stress, hyperactive response of the patient in the presence of a healthcare professional and sympathetic hyperactivity (Citation10,Citation11). Although the absence of a link between white-coat hypertension (WCH) and CHD has already been settled, little is known between the WCE and myocardial ischemia, especially in RHTN patients – with high prevalence of this condition. We aimed to investigate WCE in RHTN and assess myocardial ischemia in this group of subjects.
Methods
This study was approved by the Research Ethics Committee of the local Medical School Institution (University of Campinas – Campinas, Brazil). All patients provided written informed consent before participation, and the study was carried according to the Declaration of Helsinki.
Study population
In this cross-sectional study, 129 patients regularly followed at the Outpatient Specialized Resistant Hypertension Clinic of the University of Campinas were enrolled. The subjects had to be completely characterized as having true RHTN according to the accustomed guidelines (Citation6). All subjects were followed for at least 6 months to exclude pseudoresistance (due to non-adherence or non-optimal doses of anti-hypertensive therapy) (Citation12,Citation13) and secondary causes of hypertension (Citation12,Citation13). Patients with a moderate to high risk of obstructive sleep apnea (OSA) by the Berlin questionnaire or with diagnosed OSA were excluded (Citation14).
For the analysis, subjects were divided into two groups according to the differences in BP levels in ambulatory BP monitoring (ABPM) and office BP. If the difference between 24-h ABPM and office BP measurements was higher than 20 mmHg in the systolic or higher than 10 mmHg in the diastolic BP, they were considered as having WCE (n = 63) (Citation9,Citation15). Patients not meeting these criteria were put in the non-WCE group (n = 66).
Blood pressure measurements
Office BP measurements were performed three times in each office clinic visit in the right upper arm. The patient was put in a calm environment, and measurements assessed after a 10-min rest in the sitting position with a validated digital sphygmomanometer (Omron HEM-711DLX, OMRON Healthcare Inc., Bannockburn, IL, USA), according to international guidelines (Citation16).
Assessment of 24-h ABPM was performed with a validated automatic device (Spacelabs 90217, Spacelabs Inc, Redmon, WA, USA) (Citation17), and measurements were taken every 20 min. Patients were oriented to maintain their usual daily activities. The parameters measured were: average 24-h systolic, diastolic, mean and pulse pressures, and heart rate. Data were only analyzed if there were at least 70% satisfactory measurements completed (Citation18).
Myocardial perfusion scintigraphy imaging
Image acquisition at rest began 45 min after 925–1480 MBq of Tc-99m sestamibi injection. A dual-headed camera (Millenium MG, GE Medical Systems, Milwaukee, WI) with a high-resolution collimator rotated in a 180° orbit was used and acquired 64 projections (20 s/projection). Vasodilator stress was then induced by dipyrimidole and new images were acquired and interpreted by two experienced observers through a semi-quantitative visual interpretation of myocardial perfusion was in short-axis and vertical long-axis tomograms (Citation19). Myocardial perfusion defects were described after a quantitative evaluation with a 17-myocardial segment approach, where each segment is graded through a 5-point scoring system (Citation19). Images were compared at rest and after stress, and perfusion abnormalities were considered as having: (i) myocardial scarring when the perfusion defects were maintained on the two phases and (ii) myocardial ischemia if the perfusion defects appeared or increased after stress tomograms (Citation19,Citation20).
Statistical analysis
The analyses were performed using SigmaPlot software (Systat Software, Inc.v.12, Chicago, IL, USA). Data are shown as mean± standard deviation, or median (interquartile range) depending on data distribution, and categorical data were presented as percentages. Comparisons of groups were performed with Student's t-test or Mann–Whitney U, and categorical variables were compared by chi-square. Binary logistic regression was used to assess the prediction of myocardial ischemia by independent variables. The level of significance (α) accepted was 0.05.
Results
Patients enrolled had been diagnosed with RHTN for 17 ± 9 years and had a follow-up of 7 ± 2 years. Thirty-six patients (28%) had myocardial ischemia in the scintigraphy. There were no differences in age, gender, presence of risk factors and medication use between groups ().
Table I. Characteristics of resistant hypertension (RHTN) patients with white-coat effect (WCE) and without (non-WCE).
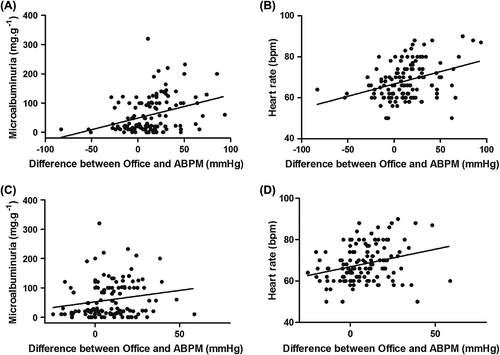
The WCE group presented: (i) higher prevalence of myocardial ischemia (49.2% vs 7.6%, p < 0.001), (ii) more microalbuminuria (MA; 60.3% vs 36.4%, p = 0.01), and (iii) higher 24-h ABPM heart rate (72 [64–80] vs 64 [60–69], p < 0.001), compared with those subjects without WCE. The differences between office BP and ABPM measurements, both systolic and diastolic, correlated with MA (r = 0.34, p < 0.001 and r = 0.28, p = 0.001) and heart rate (r = 0.36, p < 0.001 and r = 0.18, p < 0.03), respectively ().
Figure 1. Correlations between the intensity of the white-coat effect (WCE), differences in office blood pressure (BP) and ambulatory BP monitoring (ABPM), and microalbuminuria and heart rate. Panels (A) and (B) systolic blood pressure. Panels (C) and (D) diastolic blood pressure.
On a binary logistic regression, adjusting for body mass index (BMI), age and gender, heart rate was a predictor of WCE (OR = 1.10, 95% CI 1.04–1.15; p < 0.001), but MA was not (OR = 1.8, 95% CI 0.8–3.9; p = 0.15). On a second model of logistic regression, adjusting for the same variables, the presence of WCE was considered an independent predictor of myocardial ischemia (OR = 14.7, 95% CI 4.8–44.8; p < 0.001).
Discussion
We assessed myocardial ischemia and WCE in a population of true resistant hypertensive subjects. Our main findings were that there is a high prevalence of the WCE in RHTN (49%), and that in these patients the presence of myocardial ischemia is higher than in those without WCE. Indeed, the renal target organ damage assessed by MA and heart rate were also greater in WCE. Thus, the WCE was predicted by elevated heart rate and was considered an independent predictor of myocardial ischemia in patients with RHTN.
The definition of WCE can be a little confusing. The difference from WCH is that the latter is a phenomenon where the untreated patient presents an elevated office BP level that normalizes in 24-h ABPM – being a hypertensive status due to the presence of a healthcare professional. Conversely, WCE occurs when there is a decrease in awake BP levels on the 24-h ABPM compared with office BP evaluation but not necessarily normalizing it (Citation15).
The importance of assessing BP through 24-h ABPM in hypertensive patients is well established (Citation21–23); however, when patients present elevated BP levels (above goal) both in ABPM and office BP measurements, the impact of presenting the white-coat phenomenon is still obscure, especially in RHTN.
Differently from what studies have found regarding the decreased incidence of cardiovascular outcomes in patients with WCH compared with the masked or maintained hypertension (Citation24), we found that MA and myocardial ischemia are more frequent in WCE. However, we analyzed patients with RHTN (therefore a more afflicted set of hypertensive patients), and those that were still considered resistant in 24-h ABPM evaluation – presenting only the phenomenon of WCE, but not the hypertension caused in the presence of a white-coat professional.
When assessing patients with WCH, it has been shown that myocardial ischemia is less prevalent than in hypertensive subjects (Citation25). In contrast, in our sample, myocardial ischemia was not only more often present, but was also a predictor of WCE. This apparent difference in findings could be because our patients did not present WCH, and the WCE along with the presence of RHTN would impact more on the myocardium perfusion abnormalities.
MA was found to be associated with the WCE in our sample. Recently our research group has found that the MA was not statistically greater in patients with the WCE phenomenon (Citation9); however, the power of the performed test at that time did not reach 80%. Even though the presence of MA was associated with WCE, MA was not considered a predictor of WCE in our sample.
In our sample, the WCE group presented higher 24-h heart rate measure, and it was even a predictor of having WCE. This can be explained due to an increased autonomic imbalance in this group of patients (Citation26). The WCE phenomenon has been associated to occur in patients with increased sympathetic and decreased parasympathetic activities (Citation27,Citation28).
Myocardial ischemia was also more prevalent when WCE was present, and the occurrence of this phenomenon was considered a predictor of myocardial ischemia in RHTN. The link between coronary artery disease and that same autonomic imbalance has already been shown (Citation29). Since this sympathetic hyperactivity may be enhanced in WCE, we can infer that the higher prevalence of myocardial ischemia in WCE may be explained by the autonomic imbalance in this group of RHTN subjects.
As a limitation to our study, this is a cross-sectional observational study, limiting causal relationship. Moreover, the myocardial ischemia evaluation in these patients, although accurate, has inherent flaws, in particular regarding false negatives because of balanced ischemia (Citation30). Also, interpretation of the 24-h heart rate has to be taken cautiously, since the patients were not at rest, but maintaining their daily activities, which certainly differ between subjects.
In conclusion, the WCE has higher prevalence in RHTN, and its presence predicts myocardial ischemia in this specific group of patients. MA – a renal hypertensive damage – is also altered in WCE, and the intensity of the WCE correlates linearly and positively to the levels of MA. Our suggestion is that in RHTN patients with WCE, special attention should be given to CHD, in order to early diagnose any alteration and program the best therapy. Further larger and longitudinal studies should be carried out to test these findings in the settings of prognostic conclusions.
Acknowledgements
This study was supported by the State of São Paulo Research Foundation (Fapesp) and National Council for Scientific and Technological Development (CNPq), Brazil.
Declaration of interest: The authors declare that they have no conflicts of interest.
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics – 2013 update. A report from the American Heart Association. Circulation. 2013;127:E6–E245.
- Macmahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood-pressure, stroke, and coronary heart-disease. 1. Prolonged differences in blood-pressure – Prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension.2011;57:1076–1080.
- Acelajado MC, Pisoni R, Dudenbostel T, Dell’Italia LJ, Cartmill F, Zhang B, et al. Refractory hypertension: Definition, prevalence, and patient characteristics. J Clin Hypertens. 2012;14:7–12.
- de la Sierra A, Banegas JR, Oliveras A, Gorostidi M, Segura J, de la Cruz JJ, et al. Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J Hypertens. 2012;30:1211–1216.
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: Diagnosis, evaluation, and treatment – A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419.
- Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422–1428.
- Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant?Am J Hypertens. 2001;14:1263–1269.
- Figueiredo VN, Martins LC, Boer-Martins L, de Faria APC, Moraes CD, Santos RC, et al. The white coat effect is not associated with additional increase of target organ damage in true resistant hypertension. Med Clin-Barcelona. 2013; 140:1–5.
- Gualdiero P, Niebauer J, Addison C, Clark SJ, Coats AJS. Clinical features, anthropometric characteristics, and racial influences on the ‘white-coat effect’ in a single-centre cohort of 1553 consecutive subjects undergoing routine ambulatory blood pressure monitoring. Blood Press Monit. 2000;5:53–57.
- Lantelme P, Milon H, Gharib C, Gayet C, Fortrat JO. White coat effect and reactivity to stress – Cardiovascular and autonomic nervous system responses. Hypertension. 1998;31: 1021–1029.
- de Souza WA, Sabha M, Favero FD, Bergsten-Mendes G, Yugar-Toledo JC, Moreno H. Intensive monitoring of adherence to treatment helps to identify “true” resistant hypertension. J Clin Hypertens. 2009;11:183–191.
- De Souza WA, Yugar-Toledo JC, Bergsten-Mendes G, Sabha M, Moreno H. Effect of pharmaceutical care on blood pressure control and health-related quality of life in patients with resistant hypertension. Am J Health-Syst Ph. 2007;64:1955–1961.
- Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105:1135–1139.
- Pickering TG, Gerin W, Schwartz AR. What is the white-coat effect and how should it be measured?Blood Press Monit. 2002;7:293–300.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252.
- Groppelli A, Omboni S, Parati G, Mancia G. Evaluation of noninvasive blood-pressure monitoring devices Spacelab-90202 and Spacelab-90207 versus resting and ambulatory 24-hour intraarterial blood-pressure. Hypertension. 1992;20: 227–232.
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219.
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol. 2002;9:240–245.
- Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death – Differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543.
- Hansen TW, Kikuya M, Thijs L, Bjorklund-Bodegard K, Kuznetsova T, Ohkubo T, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: A meta-analysis of 7030 individuals. J Hypertens. 2007;25:1554–1564.
- Grassi G, Bombelli M, Seravalle G, Brambilla G, Dell’Oro R, Mancia G. Role of ambulatory blood pressure monitoring in resistant hypertension. Curr Hypertens Rep. 2013;15: 232–237.
- Dolan E, Stanton AV, Thom S, Caulfield M, Atkins N, McInnes G, et al. Ambulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients – An Anglo-Scandinavian cardiac outcomes trial substudy. J Hypertens. 2009;27:876–885.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: A meta-analysis. J Hypertens. 2007;25: 2193–2198.
- Nalbantgil I, Onder R, Nalbantgil S, Yilmaz H, Boydak B. The prevalence of silent myocardial ischaemia in patients with white-coat hypertension. J Hum Hypertens. 1998;12:337–341.
- Boer-Martins L, Figueiredo VN, Demacq C, Martins LC, Consolin-Colombo F, Figueiredo MJ, et al. Relationship of autonomic imbalance and circadian disruption with obesity and type 2 diabetes in resistant hypertensive patients. Cardiovasc Diabetol. 2011;10.
- Fagard RH, Stolarz K, Kuznetsova T, Seidlerova J, Tikhonoff V, Grodzicki T, et al. Sympathetic activity, assessed by power spectral analysis of heart rate variability, in white-coat, masked and sustained hypertension versus true normotension. J Hypertens. 2007;25:2280–2285.
- Neumann SA, Jennings JR, Muldoon MF, Manuck SB. White-coat hypertension and autonomic nervous system dysregulation. Am J Hypertens. 2005;18:584–588.
- Evrengul H, Tanriverdi H, Kose S, Amasyali B, Kilic A, Celik T, et al. The relationship between heart rate recovery and heart rate variability in coronary artery disease. Ann Noninvas Electro. 2006;11:154–162.
- Aarnoudse WH, Botman KJ, Pijls NH. False-negative myocardial scintigraphy in balanced three-vessel disease, revealed by coronary pressure measurement. Int J Cardiovasc Intervent. 2003;5:67–71.
