Abstract
Data examining cardiovascular (CV) risk factors in renal transplant recipients (RTRs) and their contribution to the disparity in graft survival between African American (AA) patients and non-AAs is limited. A single-center, retrospective analysis of 1003 adult RTRs from January 1, 2000 to May 1, 2008 to inspect the impact of race on post-transplant CV events, treatment of CV risk factors and their independent influence on graft outcomes was performed. AAs experienced a higher incidence of late graft loss, with 1- and 5-year graft survival rates of 93% and 76% vs 95% and 84% in the non-AA group, respectively. AA patients had a higher prevalence of hypertension (HTN) and diabetes mellitus (DM) and demonstrated reduced control of DM post-transplant (AA 74% vs non-AA 82%, p = 0.053). Multivariate analysis for graft survival indicated acute rejection, delayed graft function (DGF) and incidence of CV events were significant risk factors for graft failure, while the use of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) and 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors were protective. In conclusion, after controlling for CV risk factors and events, race did not have an independent effect on outcomes, suggesting CV risk factors and events contribute to this disparity. Clinical summary. AAs experienced a higher rate of graft failure and CV events; after adjusting for multiple immunological and CV risk factors, race no longer remained an independent risk factor for post-transplant CV events or graft failure; although disparities in post-transplant outcomes remain, race alone does not account for the disparity; the racial disparity is due to the higher incidence of DGF and acute rejection, as well as traditional CV risk factors, including HTN and DM.
Introduction
Despite advancements in immunosuppressive strategies, diagnostic testing for allograft rejection and more potent treatment regimens for infectious complications, African American (AA) renal transplant recipients (RTRs) continue to experience a disproportionately higher incidence of graft failure, a disparity that becomes even more striking 3 years post-transplant (Citation1,Citation2). Multiple studies conducted in RTRs have proposed a number of immunological and socio-economic risk factors possibly explaining this disparity. However, the actual underlying factors involved in this disparity and where to focus efforts to eliminate this inequality are unclear (Citation3–6).
Death with a functioning renal graft secondary to a cardiovascular (CV) event was the leading cause of graft loss, accounting for as much as 30% of early deaths and 75% of deaths after 1-year post-transplant (Citation7–9). The high incidence of these events post-transplant may be attributed to a patient's inherent risks, comorbidities, family history and lifestyle, along with additional acquired transplant specific risk factors (Citation10,Citation11). There are a number of studies that demonstrate AAs are more likely to develop chronic renal insufficiency and end-stage renal disease secondary to poorly controlled diabetes mellitus (DM) and hypertension (HTN), two important risk factors for the development of CV disease (Citation12,Citation13).
Although there is evidence demonstrating that HTN, DM and dyslipidemia impact patient and graft survival, there is a paucity of data analyzing the influence of CV risk factors on the disparities seen in graft outcomes in the AA compared with the general renal transplant population. This study's primary objective was to determine whether the racial disparity in graft survival could be explained by potential differences in CV risk factors.
Methods
Study design
This was a single-center, retrospective analysis to evaluate adults that received a solitary kidney transplant between January 1, 2000 and May 1, 2008 approved by the Medical University of South Carolina (MUSC) Institutional Review Board. The primary objective was to determine how the incidence and control of HTN, DM and dyslipidemia impact CV event rates, graft survival and mortality differently in the AA, compared with non-AA patients. Primary outcome measures included incidence of CV events, allograft survival and mortality.
Patients
All adult patients (> 18 years of age) that received a solitary renal transplant and follow-up care at our center were included in this analysis. Excluded patients include: < 18 years of age at the time of transplant, a recipient of an extrarenal allograft, or those lost to follow-up during the study period. Patients were defined as lost to follow-up for the following reasons: (i) patient relocated and was no longer followed by MUSC physicians, or (ii) patient no longer attended annual transplant follow-up visits for unknown reasons.
Data collection
All data analyzed were collected manually through a comprehensive review of medical charts and electronic records.
Definitions
Donors were classified as living, standard cadaveric and extended criteria cadaveric donor. An extended criteria donor (ECD) was defined as any donor over age 60, or a donor between 50 and 59 years old with two or more of the following risk factors: (i) cerebrovascular accident (CVA) as cause of death (COD), (ii) history of HTN, or (iii) terminal serum creatinine (SCr) > 1.5 mg/dl. Acute rejection had to be biopsy proven and treated; biopsy had to be reported as borderline at minimum, as defined by the Banff ’97 criteria. Delayed graft function (DGF) was defined as the need for dialysis within the first 7 days following renal transplantation.
CV risk factors assessed in the study included pre-transplant HTN, DM and dyslipidemia; smoking status; new-onset DM after transplant (NODAT); and obesity. Goals for treatment of HTN were based on JNC-VII guidelines. Dyslipidemia goals and recommendations for treatment were based on the report from NCEP III. Assessment of successful treatment to goal for DM was based on 2008 ADA criteria that recommends a hemoglobin (Hgb) A1c level of < 7%, which correlates with mean plasma glucose levels of approximately 170 mg/dl. Due to the lack of Hgb A1c levels recorded in our patient population, a mean plasma glucose level of all annual time points was utilized for treatment analysis. NODAT was determined in one of the following ways; (i) notation in the patient's medical record of NODAT or (ii) the chronic use of insulin or oral antiglycemic agent, initiated after transplantation, indicated in patient's medical record. For assessment of disease state control, annual blood pressure (BP), low-density lipoprotein (LDL) and blood glucose levels were summed and averaged in order to obtain a mean value for each patient. Control of HTN, DM and dyslipidemia was assessed in order to determine the effect of control of these disease states on incidence of CV events and graft failure. Patient self-reporting that was notated in the patient's medical record determined smoking status. Patient's smoking status was not assessed post-transplant any time during the study follow-up period. Obesity was determined by two methods: (i) noted as a part of the patient's past medical history in medical record; (ii) body mass index (BMI) calculation based upon patient's weight and height on date of transplant and categorized. CV events occurring post-transplant were categorized as occurrence of acute myocardial infarction (AMI), stroke, coronary artery bypass graft (CABG), catheterization and other, which included hospitalization due to heart failure exacerbation, peripheral vascular disease complications and arrhythmias.
Statistical analysis
This analysis examined the impact of the AA race on the incidence and control of CV risk factors, and the effect of those risk factors on CV events, graft survival and mortality. Groups were compared using both univariate and multivariate analysis. Nominal data was compared using chi-square test, and continuous data was compared using the Student's t-test or Mann–Whitney U, when appropriate. Kaplan–Meyer survival analysis was used to compare AA patients to non-AA patients for each primary outcome using the log rank test to determine statistical significance. Significance levels were defined as < 0.05.
For multivariate analysis and modeling, Cox Proportional Hazard Regression model was utilized, and each disease state that impacts CV event rates (HTN, dyslipidemia and DM) was analyzed separately. Initially, the following covariates were considered for inclusion in the multivariate analysis model: demographics of: age, race, gender, BMI, donor status (living, standard deceased donor or ECD deceased donor), and donor race; transplant characteristics, including: panel-reactive antibodies (PRA) ≥ 80%, human leukocyte antigen (HLA) mismatches, induction therapy, and outcomes of acute rejection and DGF; baseline surrogate values (BP, lipids, HgbA1C); history of diseases of CV risk, including HTN, DM, and dyslipidemia, as well as NODAT; smoking status; and all treatment modalities, grouped as mentioned previously, including antihypertensives, diabetic medications and antihyperlipidemic agents. The analysis used four separate dependent variables: (i) ability to reach goal treatment levels (i.e. at goal BP, at goal LDL, at goal HgbA1C or blood glucose); (ii) post-transplant CV event rates; (iii) kidney allograft failure; and (iv) death. Data was analyzed using SPSS version 17.0 (SPSS, Chicago IL).
Results
Patient characteristics
A total of 1179 adult patients were transplanted at MUSC during the observation period. Excluded subjects included 19 patients due to age less than 18 years old, 30 patients who were multi-organ transplant recipients and 127 recipients with a significant amount of missing data that precluded inclusion during the observation period. Those patients with missing data were compared with those included in study analysis in order to determine any possible disparities between patients included in analysis and those lost to follow-up. Of those patients lost to follow-up, 67 patients were AA and 59 were non-AA. A higher percentage of graft failure and patient death occurred in those patients lost to follow-up; however, neither outcome showed significance in racial comparisons.
After exclusions, 1003 patients were evaluated, 576 were AA and 427 were non-AA patients (). Of note, the AA RTRs mean age at time of transplant was 48 ± 13, compared with 50 ± 14 years old in the non-AA group (p = 0.011). The mean length of follow-up was 3.7 ± 2.4 years in AA patients and 4.0 ± 2.4 years in the non-AA population (p = 0.050). AAs also had a greater number of HLA mismatches, and were more likely to receive a transplant from a deceased donor. Baseline pre-transplant disease showed difference between groups for DM (AA 35% vs non-AA 23%, p < 0.001), HTN (97% vs 94%, p = 0.032), smoking (AA 25% vs non-AA 35%, p = 0.002) and dyslipidemia (36% vs 44%, p = 0.007). AA patients were also more likely than non-AA recipients to have a family history of DM and HTN.
Table I. Patient demographics (mean, median, or percentage).
Immunosuppressive regimens are summarized in . Compared with non-AA recipients, a slightly lower percentage of AA patients used tacrolimus (FK) for maintenance immunosuppression (AA 50% vs non-AA 54%). Mean 12-h trough concentrations of FK were similar across most time points; however, concentrations were significantly higher in the AA recipients at 6 months post-transplant (AA 10.2 ng/ml vs non-AA 7.8 ng/ml, p = 0.010) and lower in AA patients at 7 years post-transplant (AA 4.1 ng/ml vs non-AA 5.5 ng/ml, p = 0.026). Comparison of CyA trough concentrations demonstrated no significant difference at any time point post-transplant.
Table II. Immunosuppressive regimens (percentage or mean).
Comorbid disease state control prior to and post-transplant, including HTN, dyslipidemia and DM, as well as incidence of primary and secondary outcomes, is summarized in . Prior to transplant, on average, AA and non-AA patients had similar systolic BP (SBP) (AA 138 mmHg vs non-AA 136 mmHg, p = 0.110); however, AAs utilized more antihypertensive medications (AA 1.24 vs non-AA 0.98, p < 0.001). After transplant, AA and non-AA patients used a relatively equal number of antihypertensive medications (AA 3.1 vs non-AA 3.2 p = 0.324). Post-transplant mean SBP values showed that 32% of AA patients and 37% of non-AA recipients (p = 0.140) had optimal control (SBP < 130 mmHg). After transplant, the non-AA population showed greater control of LDL (AA 43% vs non-AA 55%, p = 0.022), as well as slightly higher utilization of statin therapy (68% vs 70%, p = 0.449). NODAT occurred in 13% of both the AA and non-AA population, for a cumulative incidence of DM of 48% and 37% (p < 0.001), respectively. The mean glucose post-transplant in patients with DM was 147 mg/dl in the AA group and 147 mg/dl in non-AA patients (p = 0.862). Differences in control of DM and insulin use, comparing the AA populations to non-AA patients, was found to be 75% vs 73% (p = 0.654) and 36% vs 31% (p = 0.091), respectively. DGF was twice as prevalent in the AA population (17% vs 9%, p < 0.001). Acute rejection within the first 12 months and overall rejection were significantly higher in the AA population (22% vs 14%, p = 0.001; 30% vs 18%, p < 0.001, respectively). Incidence of CV events and overall patient survival did not show significant differences between the two groups across all time points. At 1 and 5 years post-transplant, the incidence of CV events was 7.1% and 14.8% in the AA population and 6.6% and 12.6% in non-AA patients (1-year, p = 0.801; 5-year, p = 0.356,), respectively. At the end of the observation period, the total incidence was 16.0% in the AA group and 13.3% in the non-AA study participants (p = 0.281). For patient survival, the rates for AA recipients at 1, 5 and 7 years were 97.2%, 92.5% and 90.8%, respectively; compared with rates of 96.5%, 92.3% and 90.9% in the non-AA group 0.581; p = 0.904; p = 1.000). Compared with non-AA recipients, graft survival was significantly lower in the AA population at all observation points (p = 0.025). At 1 and 5 years, in AA patients, graft survival was 93.7% and 84.9%, and, 94.8% and 89.0% in the non-AA group (p = 0.496; p = 0.061), respectively. At the end of the observation period, AA graft survival was 72%, compared with 78% in non-AA recipients (p = 0.025).
Table III. Post-transplant cardiovascular risk factor control and outcomes assessment (mean or percentage).
Relationships between transplant outcomes, cardiovascular risk factors and incidence of cardiovascular events
Differences in incidence of CV events during the follow-up period were analyzed using Kaplan–Meyer analysis. A total of 149 CV events, 92 in the AA population and 57 in non-AA recipients, occurred during the follow-up period. displays event free survival over the entire follow-up period. (top panel, Post-transplant cardiovascular events – 135 events) displays results of the Cox Proportional Hazard model for CV events. The initial analysis contains only demographic variables (1st column), including age (years), AA race and male gender. The second analysis added transplant and CV risk factors to the model (2nd column). The last model included the addition of medication interventions that showed potential protective effects (3rd column). The first model demonstrates that race is an independent predictor of CV outcomes; however, once transplant risk factors, CV risk factors and medications used to treat these conditions are added to the model (as displayed in the final model), race was no longer a predictor of CV event outcomes.
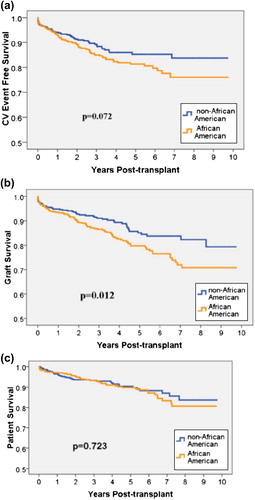
Table IV. Cox Proportional Hazard analysis of potential risk factors of primary outcomes.
Relationship between transplant risk factors, CV risk factors and renal allograft failure
Differences in graft survival between AA and non-AA RTRs for the duration of the study period are shown in . Transplant and CV risk factors, including incidence of a CV events, were analyzed to determine independent effects on graft outcomes using Cox Proportional Hazard analysis (, middle panel, Renal allograft failure – 151 events). This modeling was conducted in a similar fashion to the multivariate analysis conducted for CV event outcomes, with three distinct models. The final model included patient demographics, transplant and CV risk factors and medications used to treat these conditions. Similar to the CV events models, once demographics, transplant risk factors, and CV events and risk factors were added to the model, AA race was no longer an independent risk factor for allograft failure.
Relationships between transplant outcomes, CV risk factors and RTR mortality
RTR death was analyzed using Kaplan–Meyer Survival analysis () and Cox Proportional Hazard analysis in order to identify factors independently associated with mortality after transplantation (, bottom panel, Renal transplant recipient mortality – 92 events). Modeling was conducted in a similar fashion to the two previous outcome analyses. Results from the final model analysis showed that patients who died throughout the study period were more likely to have the diagnosis of DM, including new onset diabetes after transplant (HR = 3.03, 95% CI 1.82–4.92, p < 0.001). AA race was not a risk factor for death in any of the models.
Discussion
Results of this study demonstrate that AA RTRs continue to demonstrate inferior graft outcomes after transplant. Consistent with literature, this analysis demonstrates that AAs have higher rates of known risk factors for the development of graft loss, which are likely related to having less living donor transplants. Study results are striking when modifiable CV risk factors and CV events are included in multivariate models along with traditional transplant risk factors; the influence of race on graft failure is eliminated. This is the first large-scale study of its kind to demonstrate these results, with substantial follow-up time.
Multiple studies in the last three decades have evaluated the disparities in renal transplantation between AA and non-AA patients. However, the study presented here is the first to examine the impact race may have on the patient's ability to control CV risk factors, including HTN, DM and dyslipidemia in the kidney transplant population (Citation1,Citation3–5,Citation14–18). Several studies have analyzed the impact associated with donor and recipient characteristics, immunosuppressive regimens and outcome disparities, comparing AA patients with their non-AA counterparts (Citation3–5), while other reports attributed the graft survival disparity between Caucasian and AA patients to non-compliance, socio-economic status and access to healthcare (Citation19,Citation20). Conversely, Chakkera et al. (Citation2) analyzed outcomes of RTRs within and outside of the Department of Veteran Affairs healthcare system and provided evidence that the disparity in outcomes based on race persists in a universal healthcare setting where socio-economic status is a non-issue. Therefore, further investigations of the possible causes of this disparity, such as CV disease state management in the post-RTR population, were warranted.
RTRs are burdened with a significant risk of CV disease prior to transplant, and diseases such as HTN and DM are the cause of end stage renal disease. Reports have shown that CV disease mortality accounts for 30% of early and 75% of late post-transplant deaths (Citation9,Citation21). Numerous studies have analyzed the cause of non-fatal CV events and CV disease mortality after transplant, and risk factors for these events are well documented and include HTN, DM, dyslipidemia, obesity and history of tobacco use (Citation1,Citation3,Citation4,Citation21).
In our study population, incidence of HTN was the most prevalent CV disease risk factor, regardless of race, affecting 97% of AA patients and 94% of non-AA transplant recipients. Multiple studies have been conducted to determine the impact HTN has on graft and patient survival, specifically incidence of CV events; however, very few include an adequate number of AA RTRs and even fewer examine the effect of optimal treatment and medication intervention. While our study results did not show that HTN is an independent risk factor for CV events or allograft failure, multivariate analysis did demonstrate a significant positive relationship between exposure to ACEI or beta-blocker therapy and graft survival and mortality. This is an interesting finding, consistent with data from the general population, and warrants further investigation in the renal transplant population (Citation22–25).
The development of NODAT, for which the increased risk for post-transplant CV events is well documented, was equal between groups, each with incidence of 17% (Citation17,Citation26). However, AA patients were significantly more likely to have DM pre-transplant. The influence of DM on graft outcomes may account for one of the most important factors associated with the racial disparities seen in the kidney transplant population. In multivariate analyses, both pre-transplant DM and NODAT were significant risk factors of post-transplant CV events, graft failure and patient death. These findings suggest that the mere incidence of DM, regardless of pre-transplant diagnosis or incidence of NODAT, may put patients at increased risk of post-transplant CV events and consequent graft failure or death. The relationship between race, immunosuppression and DM merits further study in order to determine the best approach to achieve optimal patient and graft outcomes in this challenging population.
The incidence of dyslipidemia in our study population was low, at approximately 39%. This may be due to the large number of AA patients, which had a significantly lower rate of dyslipidemia, compared with non-AA patients. Studies have found a relationship between hypercholesterolemia, progressive worsening of graft function and risk of CV event post-transplant (Citation27–29). For treatment of dyslipidemia following renal transplantation, 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or statins, have become the therapy of choice (Citation30). Our study shows that statin therapy leads to significant risk reduction for graft failure and patient death regardless of race or lipid lowering effect. Of note is that AA patients were less likely to have dyslipidemia, and therefore, less likely to receive statin therapy. The positive effects of statin therapy on graft and patient survival were independent of serum lipid levels and AA patients may be at a disadvantage due to the decreased use of statins.
In summary, our results demonstrate that AA kidney transplant patients continue to have lower graft survival rates. Traditionally identified risk factors are present at a higher rate in AA recipients, including longer waiting times on the transplant list, more HLA mismatches, higher acute rejection rates, more DGF related to longer cold ischemic times, and less living donors. The effects of modifiable and non-modifiable CV risk factors and events also significantly contribute to the reduced graft outcomes seen in AA recipients. The incidence and treatment of DM appears to be one of the most significant risk factors for AA patients. Most interestingly, analysis across all models developed to identify variables associated with clinical outcomes demonstrates that race consistently falls out as a risk factor once all traditional transplant risk factors and CV risk factors are included in the analyses. This finding suggests we may have identified most of the important factors associated with inferior graft outcomes in AA patients.
While this study is unique in its depth and type of analyses for kidney transplant outcomes, it is not without its limitations. Retrospective data collection inevitably leads to missing information in patient medical records and potential reporting bias. Reporting of CV events may be incomplete due to patients being treated at other hospitals or due to incomplete reporting after treatment at our center. Long-term, because patients are only seen annually in our outpatient clinic, lab values and BP values were only collected annually after the first year post-transplant.
Conclusions
AAs experienced a higher rate of graft failure and CV events. However, after adjustment for multiple immunological and CV risk factors, race no longer remained an independent risk factor for post- transplant CV events or graft failure. These results suggest that while disparities in post-transplant outcomes remain, race alone does not account for the disparity. Instead, the racial disparity is due to the higher incidence of DGF and acute rejection, as well as traditional CV risk factors, including HTN and DM in this cohort of patients.
Financial disclosure
A Novartis Pharmaceutical Corporation Outcomes Study Grant helped fund this project. The sponsors had no role in the design and conduct of the study. The data was collected, analyzed and interpreted by the authors listed without bias. The manuscript was not prepared with any pressure or expectation from the study sponsor. The preliminary results were shared with an educational panel from Novartis in order to meet the agreed upon deliverables. The authors declare no competing financial interests.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- de Mattos AM, Prather J, Olyaei AJ, Shibagaki Y, Keith DS, Mori M, et al. Cardiovascular events following renal transplantation: Role of traditional and transplant-specific risk factors. Kidney Int. 2006;70:757–764.
- Chakkera HA, O’Hare AM, Johansen KL, Hynes D, Stroupe K, Colin PM, et al. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol. 2005;16:269–277.
- Hardinger KL, Stratta RJ, Egidi MF, Alloway RR, Shokouh-Amiri MH, Gaber LW, et al. Renal allograft outcomes in African American versus Caucasian transplant recipients in the tacrolimus era. Surgery. 2001;130:738–745; discussion 45–47.
- Foster CE 3rd, Philosophe B, Schweitzer EJ, Colonna JO, Farney AC, Jarrell B, et al. A decade of experience with renal transplantation in African-Americans. Ann Surg. 2002;236: 794–804; discussion 805.
- Light JA, Barhyte DY, Lahman L. Kidney transplants in African Americans and non-African Americans: Equivalent outcomes with living but not deceased donors. Transplant Proc. 2005;37:699–700.
- Eckhoff DE, Young CJ, Gaston RS, Fineman SW, Deierhoi MH, Foushee MT, et al. Racial disparities in renal allograft survival: A public health issue? J Am Coll Surg. 2007;204:894–902; discussion 903.
- Sam R, Leehey DJ. Improved graft survival after renal transplantation in the United States, 1988 to1996. N Engl J Med.2000;342:1837–1838.
- Axelrod DA, McCullough KP, Brewer ED, Becker BN, Segev DL, Rao PS. Kidney and pancreas transplantation in the United States, 1999-2008: The changing face of living donation.Am J Transplant.2010;10(4 Pt 2):987–1002.
- Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82:603–11.
- Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to know? Where do we go from here? Special report from the National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S1–199.
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277: 1293–1298.
- Fellstrom B. Risk factors for and management of post- transplantation cardiovascular disease. BioDrugs. 2001;15: 261–278.
- Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA. 2002; 288:2421–2431.
- Humar A, Kerr SR, Ramcharan T, Gillingham KJ, Matas AJ. Peri-operative cardiac morbidity in kidney transplant recipients: Incidence and risk factors. Clin Transplant. 2001;15: 154–158.
- Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure. Collaborative Transplant Study. Kidney Int. 1998;53:217–222.
- Mange KC, Cizman B, Joffe M, Feldman HI. Arterial hypertension and renal allograft survival. JAMA. 2000;283: 633–638.
- Cosio FG, Kudva Y, van der Velde M, Larson TS, Textor SC, Griffin MD, et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–2421.
- Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62: 1440–1446.
- Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280:1148–1152.
- Kalil RS, Heim-Duthoy KL, Kasiske BL. Patients with a low income have reduced renal allograft survival. Am J Kidney Dis. 1992;20:63–69.
- Andreoni KA, Brayman KL, Guidinger MK, Sommers CM, Sung RS. Kidney and pancreas transplantation in the United States, 1996-2005. Am J Transplant.2007;7(5 Pt 2): 1359–1375.
- Saseen JJ, MacLaughlin EJ, Westfall JM. Treatment of uncomplicated hypertension: Are ACE inhibitors and calcium channel blockers as effective as diuretics and beta-blockers? J Am Board Fam Pract. 2003;16:156–164.
- Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, et al. Preserving renal function in adults with hypertension and diabetes: A consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661.
- Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:1004–1010.
- Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–2082.
- Weir MR, Fink JC. Risk for posttransplant Diabetes mellitus with current immunosuppressive medications. Am J Kidney Dis. 1999;34:1–13.
- Kasiske BL. Cardiovascular disease after renal transplantation. Semin Nephrol. 2000;20:176–187.
- Wissing KM, Abramowicz D, Broeders N, Vereerstraeten P. Hypercholesterolemia is associated with increased kidney graft loss caused by chronic rejection in male patients with previous acute rejection. Transplantation. 2000;70:464–472.
- Aakhus S, Dahl K, Wideroe TE. Cardiovascular morbidity and risk factors in renal transplant patients. Nephrol Dial Transplant. 1999;14:648–654.
- Strippoli GF, Navaneethan SD, Johnson DW, Perkovic V, Pellegrini F, Nicolucci A, et al. Effects of statins in patients with chronic kidney disease: Meta-analysis and meta- regression of randomised controlled trials. BMJ. 2008;336: 645–651.
