Abstract
The aim of the study was to examine the associations among plasma total homocysteine (tHcy) and blood pressure (BP) stages and brachial–ankle pulse wave velocity (ba-PWV) in a Chinese rural community population. In this cross-sectional study, 2148 rural community subjects with normotension and mild hypertension (HTN) were classified into four groups according to ba-PWV level. Multivariate regression showed that ba-PWV was significantly and independently correlated with tHcy (β = 5.32, p < 0.001) in the entire study population. Moreover, ba-PWV showed a significant increase with increasing plasma tHcy level in subjects with both high normal BP and grade 1 HTN (p < 0.05). Compared with optimal BP stage, ba-PWV was significantly associated with high normal BP stage (β = 193, p < 0.001) and grade 1 HTN (β = 413, p < 0.001).There was a statistical interaction effect between high normal BP stage and optimal BP stage (p = 0.045). The similar result was found between subjects with optimal BP and those with grade 1 HTN (p = 0.037). In conclusion, tHcy was independently correlated with ba-PWV in subjects with high normal BP and grade 1 HTN. High normal BP and grade 1 HTN may worsen the impact of tHcy on arterial stiffness in a Chinese rural community population.
Key Words::
Introduction
Increased arterial stiffness is an important determinant of cardiovascular risk (Citation1–3). Arterial stiffness can be measured by pulse wave velocity (PWV), which is regarded as an important indicator suggested by the current guidelines (Citation4). Previous studies showed that PWV as an indication of arterial stiffness is a critical maker of cardiovascular events (Citation5–7). Elevated total homocysteine (tHcy) has emerged as an independent cardiovascular diseases (CVD) risk factor (Citation8,Citation9). To date, there have been a considerable number of clinical trials to assess the association between Hcy and PWV, but these earlier studies yielded inconsistent results (Citation10–15). Some reported that plasma Hcy was not associated with PWV (Citation10–13), while others do (Citation14,Citation15), specifically in hypertensive adults. It is accepted that patients with both hypertension (HTN) and hyperhomocysteinemia were more likely to have CVD and cerebrovascular diseases. However, the mechanisms underlying the interaction between blood pressure (BP) and Hcy level in CVD remain less clear. Furthermore, to the best of our knowledge, few studied have assessed the role of different BP levels on the relationship between Hcy and PWV in Asian population (Citation16,Citation17). Accordingly, we designed a cross-sectional study to examine the association among plasma tHcy and BP stages and brachial–ankle PWV (ba-PWV) in a Chinese rural community population.
Materials and methods
Study Population
The study population was enrolled from the China Stroke Primary Prevention Study (CSPPT, clinicaltrials.gov identifier: NCT00794885) (Citation18). The CSPPT is a multicenter randomized controlled trial designed to confirm that enalapril maleate and folic acid tablets combined is more effective in preventing stroke among patients with HTN when compared with enalapril maleate alone. Details regarding inclusion/exclusion criteria, treatment assignment and outcome measures of the trial have been described (Citation19). Briefly, we included 2148 participants with normotension and mild HTN from Lianyungang of Jiangsu province and Anqing of Anhui province (June 2013–August 2013) who were Chinese rural community residents.
The inclusion criteria were as follows: (i) aged 45–75 years and (ii) seated systolic BP (SBP) < 160 mmHg and/or diastolic BP (DBP) < 100 mmHg at both of the two screening visits (with at least 24 h between visits).
Exclusion criteria included a reported history of myocardial infarction, stroke, heart failure, cancer, serious mental disorder, moderate or severe HTN (SBP ≥ 160 mmHg and/or DBP ≥ 100 mmHg), and unwillingness to participate or difficulty completing the survey. The study complied with the Helsinki Declaration and was approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China. All patients gave their written informed consent.
Anthropometric measurements and blood collection
All participants underwent routine physical examinations and interviews, which were carried out by trained medical doctors. Self-reported smoking status was categorized as current, former or never. Body weight and height were measured unclothed and without shoes in the morning. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). Venous blood samples were obtained from each subject after 12–15 h of fasting. Serum or plasma samples were separated within 30 min of collection and were stored at − 70°C.
Measurement of plasma tHcy and other biochemical indices
Plasma tHcy was measured by high-performance liquid chromatography (HPLC) using a modification of the protocol described by Araki and Sako. Total cholesterol (TC), triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) were analyzed by colorimetric enzymatic assays with the use of an automated analyzer (Roche Cobas e601, Switzerland). The estimated glomerular filtration rate (eGFR) was estimated with the re-expressed four-variable Modification of Diet in Renal Disease (MDRD) equation (Citation20). Fasting plasma glucose (FPG), uric acid (UA) and alanine aminotransferase (ALT) were also collected.
Measurement and definition of BP
Participants were in supine position and their right arms supported at heart level. Automatic digital sphygmomanometers (Omron HEM 705IT device; OmronHealth Care) were used to measure BP of the participants by trained volunteers from medical colleges. Three minutes of rest was given to the participant in between three successive readings of BP. We took an average of the second and third readings as the final BP value. The evaluation of BP was made according to the European Society of Hypertension/European Society of Cardiology guidelines for adults (Citation21).
According to BP levels in our cohort, all subjects were classified into four stages according to their BP values: I = optimal BP (SBP < 120 mmHg and DBP < 80 mmHg), II = normal BP (SBP between 120 and 130 mmHg and/or DBP between 80 and 85 mmHg), III = high normal BP (SBP between 130 and 140 mmHg and/or DBP between 85 and 90 mmHg), and IV = grade 1 HTN (SBP between 140 and 160 mmHg and/or DBP between 90 and 100 mmHg).
Brachial–ankle pulse wave velocity measurements (ba-PWV)
Ba-PWV was automatically measured by PWV/ABI instruments (form PWV/ABI, BP-203RPE; Omron-Colin, Japan) by trained volunteers from medical colleges. Occlusion and monitoring cuffs matched with oscillometric sensors were wrapped around subjects’ upper arms and the ankles, and pulse volume waveforms of the bilateral brachial and tibial arteries were recorded simultaneously to determine the time interval between the initial increase in brachial and tibial waveforms (the transit time, ΔTba). The transmission distance from the brachium to ankle was calculated according to body height. The path length from the suprasternal notch to the brachium (Lb) was obtained using the following equation: Lb = 0.2195 × height of the patient (cm)− 2.07. The path length from the suprasternal notch to the ankle (La) was obtained using the following equation: La = 0.8129 × height of the patient [cm]+ 12.33. And the ba-PWV value was calculated as the ratio of transmission distance from the brachium to ankle divided by the transit time: ba-PWV = (La− Lb)/ΔTba. To compare with other studies easily, we used the higher of the bilateral ba-PWV in our analysis. Distribution of ba-PWV with age and BP category is described and reference values for PWV are established (Citation22).
Statistical analysis
Continuous data are presented as mean± standard deviation (SD) and categorical variables are expressed as percentages. Differences in continuous variables between these quartiles were tested by one-way analysis of variance (ANOVA) and categorical variables were tested by chi-square test. The relationship between ba-PWV and tHcy or other CAD risk factors were determined using Pearson and Spearman correlation, depending on the distribution of variables. Independent determinants of ba-PWV were identified by multiple linear stepwise regression analysis (dependent variable: ba-PWV; independent variables: age, gender, smoking status, BMI, heart rate, SBP, TC, TG, HDL-C, FPG, UA, eGFR and tHcy level). For all tests, a two-tailed p-value of < 0.05 was considered statistically significant. All calculations were performed with SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL).
Results
Subject characteristics
Subjects were classified into quartiles according to ba-PWV levels: ≤ 1331, 1331–1498, 1331–1498, 1498–1755 and ≥ 1755 cm/s. General and biochemical characteristics of study population as a whole and distributed over quartiles are displayed in . Their mean age was 62.5 ± 7.5 years, there were more females (63.8%) and their average tHcy level was 14.17 ± 4.70 mmol/l. The average level of ba-PWV for all population enrolled was 1586 ± 379 cm/s. Participants with a higher level of ba-PWV were more likely to have a high level of tHcy (12.98 ± 3.51 vs 13.71 ± 4.36 vs 14.00 ± 4.39 vs 15.77 ± 5.90, p < 0.001). The mean tHcy level was 2.79 mmol/l higher in the highest quartile of ba-PWV than in the lowest quartile. Also, subjects with higher level of ba-PWV had higher levels of SBP, DBP, FBG, TG and UA (p < 0.001), and had lower eGFR (p < 0.001) than people with a lower level of ba-PWV.
Table I. Baseline characteristics of 2148 Chinese rural community people according to quartiles of brachial–ankle pulse wave velocity (ba-PWV) level.
Correlations between ba-PWV and tHcy
The associations of ba-PWV with tHcy and other variables assessed by Pearson's correlation and multivariate regression are listed in . In univariate analyses, age, heart rate, SBP, FPG, TC, TG, HDL-C, UA and tHcy were positively correlated with ba-PWV (all p < 0.001), whereas HDL-C (p = 0.048) and eGFR (p < 0.001) were negatively correlated with ba-PWV. In stepwise multivariate regression analyses (R2 = 0.525), age, male sex, SBP, heart rate, FPG and UA were significantly and positively associated with ba-PWV (all p < 0.001). Also, the tHcy concentration was positively correlated with ba-PWV in the entire study population after adjustment for age, gender and other CVD risk factors (β = 5.32, p < 0.001).
Table II. Univariate and multivariate associations of brachial–ankle pulse wave velocity (ba-PWV) with cardiovascular risk factors.
Associations among plasma tHcy, different BP stages and ba-PWV
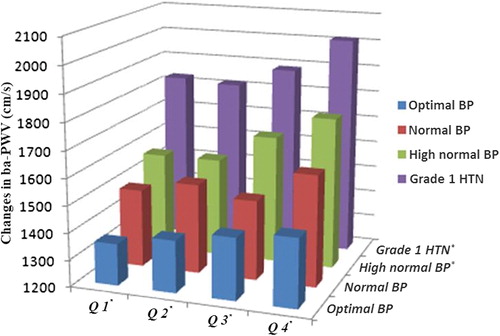
Quartiles of plasma tHcy were as follow: Q1 (≤ 11.3 mmol/l), Q2 (11.3–13.3 mmol/l), Q3 (13.3–15.8 mmol/l) and Q4 (≥ 1755 mmol/l). shows the changes in the ba-PWV in the different quartiles of tHcy and BP stages. In high normal BP and grade 1 HTN, ba-PWV significantly increased with increasing quartiles of tHcy (p < 0.05). In each of quartiles of tHcy, ba-PWV was increased with the increasing stage of BP (p < 0.05). Among all groups, ba-PWV was the highest in the Q4/Grade 1 HTN group (p < 0.05).
Multivariate analysis of relationship between ba-PWV and different BP stages
As showed in , we chose optimal BP stage as a reference. In whole cohort, ba-PWV showed positive correlations with different levels of BP: normal BP stage (β = 101, p < 0.001), high normal BP stage (β = 193, p < 0.001) and grade 1 HTN (β = 413, p < 0.001) were significantly associated with ba-PWV, independent of age, sex, smoking status, BMI, heart rate, SBP, TC, TG, HDL-C, FPG, UA, eGFR and tHcy.
Table III. Regression analysis of brachial–ankle pulse wave velocity (ba-PWV) with different blood pressure (BP) stages.
Assessment of interactions
To further explore the possible relationship between arterial stiffness and plasma tHcy, we analyzed the relationships between arterial stiffness and plasma tHcy levels in different BP stages. A statistical interaction effect was observed between high normal BP stage and optimal BP stage. Among subjects with high normal BP, there was a stronger association between plasma tHcy level and ba-PWV [β = 9.88, p < 0.001 vs β = 2.55, p = 0.215]. The impact of tHcy on ba-PWV of subjects with high normal BP was greater than that of individuals with optimal BP (β: 2.55 vs 9.88 p for interaction = 0.045). The similar result was found between subjects with optimal BP and those with grade 1 HTN, and the detailed results were presented in .
Table IV. Association between total homocysteine (tHcy) and brachial–ankle pulse wave velocity (ba-PWV) according to different blood pressure (BP) stages.
Discussion
In this present study, we assessed the association between tHcy level, BP stages and ba-PWV in a Chinese rural community population. The main findings of the study indicated that the tHcy concentration was independently and significantly associated with ba-PWV in subjects with high normal BP and grade 1 HTN.
The results presented here are at variance with those of several previous studies. Early data showed no significant association between tHcy level and PWV in a general population sample (Citation11). However, unlike the present study, the previous study focused on a young healthy people aged 20–25 years. Nakhai et al. (Citation12) investigated a middle-aged and elderly male population. They reported that PWV increased with increasing tHcy concentration in the univariate analysis, but there was not statistically significant association between circulation tHcy level and PWV after adjustment for confounders. Recently, a study on the effect of Hcy on aortic distension in healthy adults also did not find a relationship between the acute rise in Hcy and PWV (Citation13). However, both of the two studies can be limited by the relatively small sample size, which would influence the power of the study. The present study investigated a large community-based sample from China, and showed that ba-PWV significantly increased with increasing plasma tHcy level in subjects with high normal BP and grade 1 HTN. The mean tHcy level was 3 μmol/l higher in the highest quartile of ba-PWV than in the lowest quartile. Wald et al. (Citation8) have showed lowering homocysteine concentrations by 3 μmol/l from current levels would reduce the risk of ischemic heart disease by 16% (11–20%) and stroke by 24% (15–33%). Xiao et al. (Citation15) evaluated the role of plasma tHcy in arterial stiffness in Chinese hypertensive subjects. They found that mean arterial BP was independently associated with carotid–femoral PWV (cf-PWV) in both hypertensive and normotensive participants, which partly was consistent with our findings, but no significant association between tHcy and cf-PWV remained in normotensive individuals. Another retrospective study showed a close relationship between Hcy level and cf-PWV in patients with arterial HTN and normotensive controls, also consistent with our results (Citation14). However, neither reported the complex relations among tHcy and PWV and different BP stages (Citation14,Citation15). Our analysis extended the point and found significantly increased trends of ba-PWV with tHcy concentration in high normal BP stage and grade 1 HTN, which suggested that even in high normal BP stage, measuring plasma Hcy concentration may be also helpful for the early prevention and therapeutic intervention in arterial stiffness.
The second important finding of this study is that plasma tHcy level has a stronger association with ba-PWV in subjects with the high normal BP than those with the optimal BP. To our knowledge, this is the first study to investigate the interaction effect of different BP stages on arterial stiffness measured by ba-PWV in a large community-based population in China. In our analysis, statistical interaction was observed between high normal BP stage and optimal BP stage. The impact of tHcy on ba-PWV of subjects with high normal BP was stronger than that of individuals with optimal BP. The adjusted data also showed a significant interaction between grade 1 HTN and optimal BP stage in a Chinese rural community population.
The mechanisms underlying the relationship between Hcy and PWV are not entirely understood but may include endothelial dysfunction (Citation23), collagen synthesis (Citation24), increased smooth muscle cell proliferation (Citation25) and deterioration of elastic material of arterial wall (Citation26), contributing to impaired arterial compliance. Our observations suggested that high normal BP may enhances the effect of tHcy on PWV. Lengyel et al. (Citation27) have concluded that elevated serum levels of homocysteine may play a role in increased intima-media thickness (IMT) in adolescent HTN. Both IMT and ba-PWV were the marker of the vascular dysfunction, We inferred a role of hyperhomocysteinemia in the development of reversible vascular dysfunction, and there is a synergistic acceleration of vascular dysfunction in the presence of raised BP and raised tHcy. Elevated BP make the endothelium more susceptible to the deleterious effects of high level of plasma tHcy may be another explanation (Citation28). Bortolotto et al. (Citation29) has demonstrated that HTN may interact with tHcy to produce synergistic action in aortic stiffness, which partly supported our results. These findings indicated that high normal BP and grade 1 HTN played an important role in the association between tHcy and ba-PWV, and may worsen the impact of tHcy on arterial stiffness in a Chinese rural community population.
There are some limitations to the study. First, this was a cross-sectional study, and thus our research merely demonstrates an association, not a cause-and-effect relationship. Second, the study population was the middle-aged and elderly in Chinese rural areas where there was higher percentage of females, therefore there was a limitation in generalizing our results to the wider population. Third, 24-h ambulatory BP monitoring (ABPM) was superior to office BP (OBP) in identifying true hypertensive patients; no data about ABPM in our study is a potential limitation. To address this limitation, additional prospective studies with more males are required to validate the findings of the present study.
Conclusion
Our results showed that in a Chinese rural community population, plasma tHcy level was significantly associated with ba-PWV in subjects with high normal BP and grade 1 HTN. High normal BP and grade 1 HTN may worsen the impact of tHcy on arterial stiffness.
Acknowledgments
Sponsor's role: None. All authors have contributed substantially to conception and design, acquisition of data or analysis and interpretation of data, have drafted the manuscript or revised it critically and have approved the final version to be published.
Conflict of interest
None.
References
- Van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, et al. Association between arterial stiffness and atherosclerosis: The Rotterdam Study. Stroke. 2001;32:454–460.
- Jadhav UM, Kadam NN. Non-invasive assessment of arterial stiffness by pulse-wave velocity correlates with endothelial dysfunction. Indian Heart J. 2005;57:226–232.
- Yingchoncharoen T, Limpijankit T, Jongjirasiri S, Laothamatas J, Yamwong S, Sritara P. Arterial stiffness contributes to coronary artery disease risk prediction beyond the traditional risk score (RAMA-EGAT score). Heart Asia. 2012;4:77–82.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536.
- Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249.
- Paultre F, Mosca L. The relation of blood pressure to coronary heart mortality in different age groups varies by ethnicity. Am J Hypertens. 2006;19:179–183.
- Denker MG, Cohen DL. What is an appropriate blood pressure goal for the elderly: Review of recent studies and practical recommendations. Clin Interv Aging. 2013;8: 1505–1517.
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;23;325:12.
- Heinz J, Kropf S, Luley C, Dierkes J. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: A meta-analysis. Am J Kidney Dis. 2009;54:478–89.
- de Bree A, Mennen LI, Zureik M, Ducros V, Guilland JC, Nicolas JP, et al. Homocysteine is not associated with arterial thickness and stiffness in healthy middle-aged French volunteers. Int J Cardiol. 2006;113:332–340.
- Woodside JV, McMahon R, Gallagher AM, Cran GW, Boreham CA, Murray LJ, et al. Total homocysteine is not a determinant of arterial pulse wave velocity in young healthy adults. Atherosclerosis. 2004;177:337–344.
- Nakhai-Pour HR, Grobbee DE, Bots ML, Muller M, van der Schouw YT. Circulating homocysteine and large arterial stiffness and thickness in a population-based sample of middle-aged and elderly men. J Hum Hypertens. 2007;21:942–948.
- Eleftheriadou I, Grigoropoulou P, Moyssakis I, Kokkinos A, Perrea D, Toutouzas K, et al. The effect of hyperhomocysteinemia on aortic distensibility in healthy individuals. Nutrition. 2013;29:876–880.
- Vyssoulis G, Karpanou E, Kyvelou SM, Adamopoulos D, Gialernios T, Gymnopoulou E, et al. Associations between plasma homocysteine levels, aortic stiffness and wave reflection in patients with arterial hypertension, isolated office hypertension and normotensive controls. J Hum Hypertens. 2010;24:183–189.
- Xiao W, Bai Y, Ye P, Luo L, Liu D, Wu H, et al. Plasma homocysteine is associated with aortic arterial stiffness but not wave reflection in Chinese hypertensive subjects. PLoS One. 2014; doi: 10.1371/journal.pone.0085938.
- Tayama J, Munakata M, Yoshinaga K, Toyota T. Higher plasma homocysteine concentration is associated with more advanced systemic arterial stiffness and greater blood pressure response to stress in hypertensive patients. Hypertens Res. 2006;29: 403–409.
- Ruan L, Chen W, Srinivasan SR, Xu J, Sun M, Toprak A, et al. Relation of plasma homocysteine to arterial stiffness in black and white young adults (from the Bogalusa Heart Study). Am J Cardiol. 2009;103:985–988.
- Mao G, Hong X, Xing H, Liu P, Liu H, Yu Y, et al. Efficacy of folic acid and enalapril combined therapy on reduction of blood pressure and plasma glucose: A multicenter, randomized, double-blind, parallel-controlled, clinical trial. Nutrition. 2008;24:1088–1096.
- Qin X, Li J, Cui Y, Liu Z, Zhao Z, Ge J, et al. Effect of folic acid intervention on the change of serum folate level in hypertensive Chinese adults: Do methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms affect therapeutic responses? Pharmacogenet Genomics. 2012;22:421–428.
- Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772.
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357.
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350.
- Tousoulis D, Antoniades C, Marinou K, Vasiliadou C, Bouras G, Stefanadi E, et al. Methionine-loading rapidly impairs endothelial function, by mechanisms independent of endothelin-1: Evidence for an association of fasting total homocysteine with plasma endothelin-1 levels. J Am Coll Nutr. 2008;27:379–386.
- Miller A, Mujumdar V, Palmer L, Bower JD, Tyagi SC. Reversal of endocardial endothelial dysfunction by folic acid in homocysteinemic hypertensive rats. Am J Hypertens. 2002;15:157–163.
- Chiang JK, Sung ML, Yu HR, Chang HI, Kuo HC, Tsai TC, et al. Homocysteine induces smooth muscle cell proliferation through differential regulation of cyclins A and D1 expression. J Cell Physiol. 2011;226:1017–1026.
- Kumar M, Tyagi N, Moshal KS, Sen U, Kundu S, Mishra PK, et al. Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochem Int. 2008;53:214–219.
- Lengyel S, Katona E, Zatik J, Molnár C, Paragh G, Fülesdi B, et al. The impact of serum homocysteine on intima-media thickness in normotensive, white-coat and sustained hypertensive adolescents. Blood Press. 2012;2: 39–44.
- Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: From folate fortification to the recent clinical trials. Eur Heart J. 2009;30:6–15.
- Bortolotto LA, Safar ME, Billaud E, Lacroix C, Asmar R, London GM, et al. Plasma homocysteine, aortic stiffness, and renal function in hypertensive patients. Hypertension. 1999;34:837–842.