Abstract
Little is known about blood pressure in relation to circulating natriuretic peptide concentrations and gender in generally healthy adolescents. We studied 15-year-old females and males (n = 335) from the Danish site of the European Youth Heart Study (EYHS). Blood pressure was measured using a standardized protocol, sexual maturity was assessed according to Tanner stage, and as a surrogate for atrial natriuretic peptide, we measured mid-regional pro-atrial natriuretic peptide (MR-proANP) in plasma. Compared with boys, girls had lower systolic blood pressure (SBP) (mean ± SD: 109.6 ± 9.9 mmHg vs 116.9 ± 11.4 mmHg, p < 0.0001) and higher plasma MR-proANP concentrations [median (interquartile range): 42.1 pmol/l (31.9–50.2 pmol/l) vs 36.6 pmol/l (30.6–44.9 pmol/l), p = 0.0046]. When female adolescents were further subdivided according to Tanner stage, there were no differences in blood pressure and plasma MR-proANP concentrations between post-pubertal and pubertal girls (p > 0.17). In contrast, after similar subdivision, post-pubertal boys had higher SBP (mean ± SD: 117.7 ± 11.7 mmHg vs 111.4 ± 7.9 mmHg, p = 0.029) and lower plasma MR-proANP concentrations [median (interquartile range): 36.2 pmol/l (30.6–43.1 pmol/l) vs 46.4 pmol/l (30.3–51.1 pmol/l), p = 0.043] compared with pubertal boys. Given their higher SBP, boys had lower than expected plasma concentrations of MR-proANP compared with girls, and given their higher SBP, post-pubertal boys had lower than expected plasma concentrations of MR-proANP compared with pubertal boys.
Introduction
The cardiac natriuretic peptides, atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), have diuretic, natriuretic and vasodilatory properties, and are believed to play central roles in normal blood pressure regulation (Citation1,Citation2). Recent evidence, including genetically based evidence (Citation3,Citation4), seems to suggest that lower circulating natriuretic peptide concentrations could also play a central role in the development of hypertension (Citation5,Citation6).
Little is known about blood pressure in relation to circulating natriuretic peptide concentrations in healthy children and adolescents. Several studies have measured circulating natriuretic peptide concentrations (or fragments of their circulating pro-hormones) in children and adolescents (7–21), but only a few of those studies have reported blood pressure measurements (Citation17,Citation18,Citation20,Citation21). Furthermore, in most of the studies, the participating children and adolescents were recruited in hospital settings (7,8,11,13–15, 17,18,20,21) or study specimens were obtained from paediatric biobanks (Citation16). Although the studied children and adolescents only suffered from minor and uncomplicated diseases, underlying disease could potentially affect circulating natriuretic peptide concentrations. The general impression is (giving larger studies greater weight) that circulating natriuretic peptide concentrations tend to fall from birth to adolescence (7,8,11–16,19), seem to be higher in girls than in boys (Citation16) (after age 10 years) (Citation13,Citation15) and seem to be influenced by testosterone in both girls and boys (Citation16). In this perspective, teenage boys, for still not well-defined reasons, seem to have higher blood pressure than teenage girls (Citation22,Citation23). To our knowledge, no data have been published where the relationships between gender, stage of puberty, circulating natriuretic peptide concentrations and blood pressure have been investigated under well-standardized study conditions in healthy, free-living adolescents.
Therefore, we initiated this study to provide new data on the putative relationships between natriuretic peptides, gender, puberty and blood pressure in adolescents. We studied a large sample of adolescents from the Danish site of the European Youth Heart Study (EYHS) (Citation24,Citation25), which is a population-based study of cardiovascular disease (CVD) risk factors in children and adolescents. The EYHS includes standardized blood pressure measurements, assessment of sexual maturity according to Tanner stage and a biobank (Citation25), enabling us to measure plasma concentrations of mid-regional pro-atrial natriuretic peptide (MR-proANP), a stable proANP fragment (Citation26). MR-proANP measurement essentially seems to match that of the related proBNP, with one major difference: MR-proANP concentrations are much higher than those of proBNP-related peptides in non-cardiac patients and thus allow for accurate and reliable information on decreased concentrations compared with reference individuals (Citation26).
Methods
Study population
This study is a cross-sectional study using data from the Danish site of the EYHS (Citation25). The EYHS is an international population-based mixed longitudinal study that addresses biological, environmental, demographic and lifestyle correlates of CVD risk factors in children and adolescents. A description of the EYHS protocol and the sampling procedures has been reported in detail elsewhere (Citation24). In 2009–2010, a random sample of 709 15-year-old healthy adolescents was invited to take part in the Danish part of the study (Citation25). The participation rate was 59% (n = 399). We excluded 42 participants because of missing data [systolic blood pressure (SBP), diastolic blood pressure (DBP), MR-proANP, Tanner stage and fasting status], and 22 participants because they were not fasting, leaving in total 335 adolescents for analyses in the present study. The study was approved by the Regional Scientific Ethical Committee for Southern Denmark, and data were collected according to the Declaration of Helsinki. All participants gave their written informed consent.
Anthropometry and maturity
Weight, height and waist circumference (WC) were measured while the participants were wearing light clothing, without shoes, using standard techniques (Citation25). WC was measured with a metal anthropometric tape midway between the lower rib margin and the iliac crest, at the end of gentle expiration. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). The participants themselves identified their sexual maturity after having received instructions by trained staff, according to Tanner stage using a five-point scale based on pictures (Citation27). Male participants rated their maturity according to genital and pubic hair development, and girls according to breast development. Maturity stage among the participating adolescents was exclusively Tanner stage 2, 3, 4 or 5, and we collapsed maturity to a two-point ordinal variable: Tanner stage 2–3 (pubertal) and Tanner 4–5 (post-pubertal).
Biochemistry
Intravenous blood samples were drawn in the morning from an antecubital vein after participants had fasted for at least 8 h. Blood samples were separated and stored at −80°C until analysis. No samples were stored for more than 5 years. Plasma concentrations of MR-proANP were measured with a commercially available sandwich chemiluminescence immunoassay (Brahms, Hennigsdorf/Berlin, Germany) with intra-assay and interassay coefficients of variation below 2% and 6%, respectively, and a lower detection limit of 4.5 pmol/l. Based on our own laboratory experiences, we have no specific indications that the stability of MR-proANP over many years at −80°C should be a problem. We chose to measure MR-proANP concentrations rather than ANP because ANP is a very labile peptide and is prone to degradation during freeze—thaw cycles (Citation26).
Blood pressure
Blood pressure was measured by trained staff with participants in the upright sitting position after resting for 5 min (Citation24,Citation25), using a Dinamap monitor (Dinamap model XL; Kivex/Critikron, Tampa, FL, USA). Five measurements were conducted with 2 min intervals between each, and the mean of the last three measurements was used in all analyses. The Dinamap monitor has previously been validated in children against direct radial artery readings (mean error 0.24 mmHg SBP and 1.28 mmHg DBP) (Citation28).
Statistical analysis
All statistical analyses were performed using STATA 13.0 (STATA Corporation, College Station, TX). Normally distributed data are presented as mean ± standard deviation (SD) and non-normally distributed data as median (interquartile range). Group comparisons were performed using the Student's t test. Non-normally distributed data were log-transformed to fulfil the normal distribution criteria before being entered in the Student's t test. Because we also thought it of interest to explore the overall relationship between plasma MR-proANP and blood pressure in our adolescent study population, we applied multiple regression analysis to explore possible independent relationships between log-transformed MR-proANP concentrations and SBP and DBP in the entire study population, adjusting for gender, age and Tanner stage. The results of the regression analyses are presented as standardized regression coefficients with 95% confidence intervals (CIs). All statistical tests were two sided. A p value below 0.05 was considered significant.
Results
shows some general characteristics of the study population stratified by gender. Boys were (marginally) older, taller and heavier compared with girls (p < 0.004), whereas BMI was similar in both groups (p = 0.19). Boys had higher SBP (mean ± SD: 116.9 ± 11.4 mmHg vs 109.6 ± 9.9 mmHg, p < 0.0001), lower DBP (mean ± SD: 60.4 ± 6.4 mmHg vs 63.0 ± 6.4 mmHg, p = 0.0003) and lower plasma MR-proANP concentrations (median interquartile range: 36.6 pmol/l (30.6–44.9 pmol/l) vs 42.1 pmol/l (31.9–50.2 pmol/l), p = 0.0046) compared with girls.
Table I. Characteristics of participants stratified by gender.
shows some general characteristics of the participating girls, subdivided according to Tanner stage. Most girls were at Tanner stage 4 and 5. The only significant differences found between pubertal and post-pubertal girls were in weight, BMI and WC (p < 0.003), and although not statistically significant, post-pubertal girls had lower SBP and DBP and lower plasma MR-proANP concentration compared with pubertal girls.
Table II. Characteristics of participating girls stratified by pubertal stage.
displays some general characteristics of the participating boys, subdivided according to Tanner stage. Most boys were post-pubertal. Post- pubertal boys had higher SBP (p = 0.029) and lower plasma MR-proANP concentrations (p = 0.043) compared with pubertal boys, and post-pubertal boys also tended to have higher DBP (p = 0.081). Of note, there were no differences between post-pubertal and pubertal boys in age, height, weight, BMI and WC (p > 0.18).
Table III. Characteristics of participating boys stratified by pubertal stage.
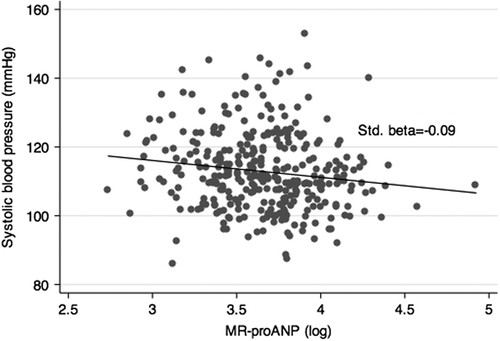
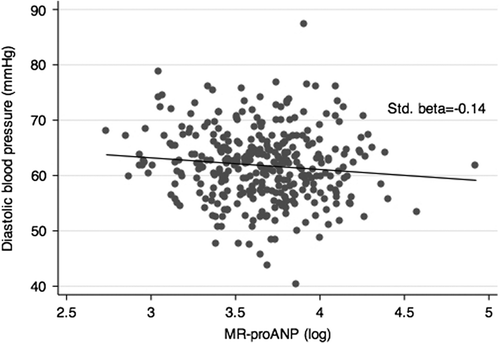
Finally, the standardized regression coefficient, including adjustment for gender, age and pubertal stage, between plasma MR-proANP and SBP was −0.09 (95% CI −0.19 to 0.01) (p = 0.086), and between plasma MR-proANP and DBP −0.14 (95% CI −0.24 to −0.03) (p = 0.011). The relationships between plasma MR-proANP and SBP and DBP are displayed as scatter diagrams in and , respectively. The respective adjusted standardized regression coefficients are also depicted in the figures.
Discussion
The principal new findings of this study were that given their higher SBP, boys had lower than expected plasma concentrations of MR-proANP compared with girls, and given their higher SBP, post-pubertal boys had lower than expected plasma concentrations of MR-proANP compared with pubertal boys. In this context, it is worthy of note that there were no differences between post-pubertal and pubertal boys with respect to demographic and anthropometric parameters. In contrast to boys, post-pubertal and pubertal girls had both similar blood pressure and circulating MR-proANP concentrations. Furthermore, contrary to known physiological blood pressure responses (29–31), in the entire study population plasma concentrations of MR-proANP unexpectedly did not increase with increasing blood pressure levels, but instead decreased. Finally, the present report is also important because it is the first to define values of MR-proANP in normal healthy adolescents.
Our results add to the growing body of evidence that lower circulating concentrations of natriuretic peptides could lead to higher blood pressure, and although our study did not include measurements of sex hormones, the results add to the growing body of evidence that higher circulating testosterone concentrations (indicated by higher stages of puberty in boys) could lead to lower circulating natriuretic peptide concentrations.
The fact that higher blood pressure was associated with lower circulating concentrations of MR-proANP was unexpected based on physiological considerations. Thus, in normal research subjects, infusion of vasopressor agents, such as angiotensin II (Citation29,Citation30), noradrenaline (Citation31) or phenylephrine (Citation30), leads to higher circulating ANP concentrations. This vasopressor-induced ANP release seems to be primarily mediated by haemodynamic changes, causing atrial stretching/distension, because additional infusions of a blood pressure-lowering agent, such as sodium nitroprusside, will decrease ANP concentrations to basal values without affecting circulating concentrations of the infused vasopressor agents (Citation31). Therefore, given their higher blood pressure, post-pubertal boys had lower than expected circulating natriuretic peptide concentrations. Because there were no differences in demographic and anthropometric parameters, otherwise known to be associated with higher blood pressure (Citation22,Citation23,Citation32,Citation33), between pubertal and post-pubertal boys, we speculate that the lower plasma MR-proANP concentrations in the post-pubertal boys could, to some degree, explain their higher blood pressure.
Only a few other studies have measured both blood pressure and natriuretic peptides (exclusively NT-proBNP) in children and adolescents (Citation17,Citation18,Citation20,Citation21). However, combining the data from the different studies did not give consistent results with respect to associations between circulating natriuretic peptide concentrations and blood pressure.
Only one other study has related circulating natriuretic peptide concentrations to Tanner stage (Citation13). However, in that study blood pressure was not measured (Citation13), and Tanner stage 1 was compared with Tanner stage 2–5, whereas we compared Tanner stage 2–3 with stage 4–5. That particular study found that plasma BNP concentrations were approximately 50% lower in pre-pubertal girls, and plasma BNP concentrations were correlated with Tanner stage in girls (r = 0.41, p = 0.001). With respect to boys, there was no significant correlation of BNP with Tanner stages (p = 0.19) and no significant difference between BNP in pre-pubertal and pubertal or mature boys (p = 0.15), although the correlation coefficient was negative (r = −0.18) and pubertal or mature boys had approximately 30% lower BNP concentrations compared with pre-pubertal boys. So, the results in boys were overall similar to our results in boys.
In this study, we have no measurements of sex hormones, but as mentioned above, our results indicate indirectly that higher circulating testosterone concentrations (indicated by higher stages of puberty in boys) could lead to lower circulating natriuretic peptide concentrations. This observation is in agreement with the medical literature based on data from both children and adolescents (Citation16), as well as data from adults (Citation34,Citation35).
Limitations and strengths
Our study is a cross-sectional study, and therefore we can only speculate about cause-and-effect relationships. It is a limitation that the overall number of pubertal girls and boys was small, and another limitation that our study is a retrospective biobank study. It is also a limitation that we have no standardized recordings of the participants’ intake of sodium, which is a central physiological regulator of ANP secretion (Citation1,Citation2). Maybe the scatter of data in and would have been less wide if we could have adjusted for sodium intake. Furthermore, we did not measure active ANP, which, however, was deliberate owing to the well-known technical difficulties of quantifying bioactive ANP (Citation26). Unfortunately, we did not measure sex hormones. Finally, we have no information on the day of the girls’ menstrual period. However, circulating ANP concentrations do not fluctuate with the cyclical changes in oestrogen and progesterone seen during the menstrual cycle (Citation36,Citation37).
Major strengths were that the participating girls and boys were healthy, free-living adolescents recruited from the general population, and that their blood pressure was measured by trained staff using standardized validated blood pressure techniques. Our study is one of a few that has measured both blood pressure and circulating natriuretic peptide concentrations in healthy adolescents (Citation17,Citation18,Citation20,Citation21). This made it possible for us to study early primary endocrine and vascular disturbances without the confounding effects of comorbidities.
Perspectives
The present study adds to the growing body of evidence that the natriuretic peptides play important roles in blood pressure regulation and that testosterone probably modulates circulating natriuretic peptide concentrations. Overall, our study provides further indications of why boys and younger men have higher blood pressure and a higher risk of hypertension compared with girls and younger women (Citation38). Finally, it is worthy of note that adolescents have been reported to have a very high salt intake (Citation39), and this could give rise to higher blood pressure, particularly among male adolescents with presumably testosterone-induced lower circulating natriuretic peptide concentrations.
Funding
The EYHS obtained funding from the following sources: the Danish Council for Strategic Research [grant number 2101-08-0058], The Danish Heart Foundation, the Danish Health Fund (Sygekassernes Helsefond). The views expressed in this paper are those of the authors and not any funding body. None of the funding bodies influenced data collection, analysis or interpretation of results.
Declaration of interest: The authors declare no conflicts of interest related to the content of this paper.
References
- Volpe M. Natriuretic peptides and cardio-renal disease. Int J Cardiol. 2014;176:630–9.
- Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol. 2014;11:402–22.
- Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–53.
- Jeppesen J, Nielsen SJ, Torp-Pedersen C, Hansen TW, Olsen MH, Berg ND, et al. Genetic variation in the natriuretic peptide system, circulating natriuretic peptide levels, and blood pressure: an ambulatory blood pressure study. Am J Hypertens. 2012;25:1095–100.
- Asferg CL, Nielsen SJ, Andersen UB, Linneberg A, Møller DV, Hedley PL, et al. Relative atrial natriuretic peptide deficiency and inadequate renin and angiotensin II suppression in obese hypertensive men. Hypertension. 2013;62: 147–53.
- Macheret F, Heublein D, Costello-Boerrigter LC, Boerrigter G, McKie P, Bellavia D, et al. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol. 2012;60:1558–65.
- Weil J, Bidlingmaier F, Döhlemann C, Kuhnle U, Strom T, Lang RE. Comparison of plasma atrial natriuretic peptide levels in healthy children from birth to adolescence and in children with cardiac diseases. Pediatr Res. 1986;2:1328–31.
- Rascher W, Bald M, Kreis J, Tulassay T, Heinrich U, Schärer K. Atrial natriuretic peptide in infants and children. Horm Res. 1987;28:58–63.
- Kikuchi K, Shiomi M, Horie K, Ohie T, Nakao K, Imura H, et al. Plasma atrial natriuretic polypeptide concentration in healthy children from birth to adolescence. Acta Paediatr Scand. 1988;77:380–4.
- Ationu A, Carter ND. Brain and atrial natriuretic peptide plasma concentrations in normal healthy children. Br J Biomed Sci. 1993;50:92–5.
- Yoshibayashi M, Kamiya T, Saito Y, Nakao K, Nishioka K, Temma S, et al. Plasma brain natriuretic peptide concentrations in healthy children from birth to adolescence: marked and rapid increase after birth. Eur J Endocrinol. 1995;133:207–9.
- Holmström H, Thaulow E, Stokke O, Lindberg H, Hall C. Serum N-terminal proatrial natriuretic factor in children with congenital heart disease. Eur Heart J. 1996;17:1737–46.
- Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003; 89:875–8.
- Rauh M, Koch A. Plasma N-terminal pro-B-type natriuretic peptide concentrations in a control population of infants and children. Clin Chem. 2003;49:1563–4.
- Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L, et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol. 2009;30:3–8.
- Saenger AK, Dalenberg DA, Bryant SC, Grebe SK, Jaffe AS. Pediatric brain natriuretic peptide concentrations vary with age and sex and appear to be modulated by testosterone. Clin Chem. 2009;55:1869–75.
- Pervanidou P, Margeli A, Akalestos A, Sakka S, Kanaka-Gantenbein C, Papassotiriou I, et al. Associations between circulating N-terminal pro-brain natriuretic peptide (NT-proBNP) and adiponectin concentrations depend on obesity level in female adolescents: gender dimorphic findings. Horm Metab Res. 2009;41:829–33.
- Pervanidou P, Akalestos A, Sakka S, Kanaka-Gantenbein C, Papassotiriou I, Chrousos GP. Gender dimorphic associations between N-terminal pro-brain natriuretic peptide, body mass index and blood pressure in children and adolescents. Horm Res Paediatr. 2010;73:341–8.
- Siervo M, Ruggiero D, Sorice R, Nutile T, Aversano M, Iafusco M, et al. Body mass index is directly associated with biomarkers of angiogenesis and inflammation in children and adolescents. Nutrition. 2012;28:262–6.
- Battal F, Ermis B, Aktop Z, Can M, Demirel F. Early cardiac abnormalities and serum N-terminal pro B-type natriuretic peptide levels in obese children. J Pediatr Endocrinol Metab. 2011;24:723–6.
- Li AM, Au CT, Zhu JY, Chan KC, Chan MH, Lee DL, et al. Plasma natriuretic peptides in children and adolescents with obstructive sleep apnea and their changes following intervention. Front Pediatr. 2014 Mar 24;2:22. doi: 10.3389/fped.2014.00022.
- Spagnolo A, Giussani M, Ambruzzi AM, Bianchetti M, Maringhini S, Matteucci MC, et al. Focus on prevention, diagnosis and treatment of hypertension in children and adolescents. Ital J Pediatr. 2013;39:20. doi: 10.1186/1824–7288-39-20.
- Bucher BS, Ferrarini A, Weber N, Bullo M, Bianchetti MG, Simonetti GD. Primary hypertension in childhood. Curr Hypertens Rep. 2013;15:444–52.
- Riddoch C, Edwards D, Page A, Froberg K, Anderssen S, Wedderkopp N, et al. The European Youth Heart Study–cardiovascular disease risk factors in children: rationale, aims, design and validation of methods. J Phys Act Health. 2005;2:115–29.
- Ried-Larsen M, Grøntved A, Ostergaard L, Cooper AR, Froberg K, Andersen LB, Møller NC et al. Associations between bicycling and carotid arterial stiffness in adolescents: The European Youth Hearts Study. Scand J Med Sci Sport. 2014 Aug 26. doi: 10.1111/sms.12296 [Epub ahead of print]
- Goetze JP, Hansen LH, Terzic D, Zois NE, Albrethsen J, Timm A, et al. Atrial natriuretic peptides in plasma. Clin Chim Acta. 2015;443:25–8.
- Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55.
- Park MK, Menard SM. Accuracy of blood pressure measurement by the Dinamap Monitor in infants and children. Pediatrics. 1987;79:907–14.
- Cargill RI, Coutie WJ, Lipworth BJ. The effects of angiotensin II on circulating levels of natriuretic peptides. Br J Clin Pharmacol. 1994;38:139–42.
- Shenker Y, Bates ER, Egan BH, Hammoud J, Grekin RJ. Effect of vasopressors on atrial natriuretic factor and hemodynamic function in humans. Hypertension. 1988;12:20–5.
- Uehlinger DE, Zaman T, Weidmann P, Shaw S, Gnädinger MP. Pressure dependence of atrial natriuretic peptide during norepinephrine infusion in humans. Hypertension. 1987;10:249–53.
- Kannel WB. Framingham study insights into hypertensive risk of cardiovascular disease. Hypertens Res. 1995;18: 181–96.
- Kannel WB, D’Agostino RB, Cobb JL. Effect of weight on cardiovascular disease. Am J Clin Nutr. 1996;63(3 Suppl): 419–22S.
- Lam CS, Cheng S, Choong K, Larson MG, Murabito JM, Newton-Cheh C, et al. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol. 2011;58:618–26.
- Chang AY, Abdullah SM, Jain T, Stanek HG, Das SR, McGuire DK, et al. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J Am Coll Cardiol. 2007;49:109–16.
- Clark BA, Elahi D, Epstein FH. The influence of gender, age, and the menstrual cycle on plasma atrial natriuretic peptide. J Clin Endocrinol Metab. 1990;70:349–52.
- Maffei S, Clerico A, Iervasi G, Nannipieri M, Del Ry S, Giannessi D, Donato L. Circulating levels of cardiac natriuretic hormones measured in women during menstrual cycle. J Endocrinol Invest. 1999;22:1–5.
- Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond). 2013;125:311–8.
- Marrero NM, He FJ, Whincup P, Macgregor GA. Salt intake of children and adolescents in South London: consumption levels and dietary sources. Hypertension. 2014;63:1026–32.