Abstract
Background. Arterial stiffness is an independent predictor of cardiovascular disease risk. However, whether genetic risk variants are associated with arterial stiffness measures, such as pulse-wave velocity (PWV), is largely unknown. Therefore, we performed a genome-wide association study (GWAS) to identify single-nucleotide polymorphisms (SNPs) associated with PWV in a Korea population. Method. Study participants consisted of 402 patients in the Yonsei cardiovascular genome center cohort. Arterial stiffness was measured as brachial–ankle pulse-wave velocity (baPWV). Genotyping was performed in 402 subjects with the Axiom™ Genome-Wide ASI 1 Array Plate containing more than 600,000 SNP markers. The findings were tested for replication in independent subjects from a community-based cohort of 1206 individuals, using a Taqman assay to include two candidate SNPs. Associations with PWV were evaluated using an additive genetic model that included age, gender, systolic blood pressure and diastolic blood pressure as covariates. GWAS and replication analyses were conducted using the measured genotype method implemented in PLINK and SAS. Results. We observed two candidate SNPs associated with baPWV in GWAS: rs7271920 (p = 7.15 × 10–9) and rs10125157 (p = 8.25 × 10–7). However, neither of these was significant in the replication cohort. Conclusion: In summary, we did not identify any common genetic variants associated with baPWV in cardiovascular patients.
Introduction
Arterial stiffness, assessed by pulse-wave velocity (PWV), is one of the risk factors for cardiovascular mortality and events (Citation1). PWV is increased in patients with hypertension (Citation2), stroke (Citation3) and coronary heart disease (Citation3), and is an independent predictor of mortality (Citation4). Therefore, understanding the mechanisms of arterial stiffness is becoming a crucial point in the prevention and early detection of cardiovascular disease (Citation5–7). Heritability of arterial stiffness is estimated to be between 0.13 and 0.54 (Citation8,Citation9), suggesting that arterial stiffness is influenced by genetic factors. Recently, two genome-wide association studies (GWAS) have been conducted, primarily in subjects of European descent (Citation10,Citation11). To date, few studies have evaluated the association between common genetic variants from GWAS and PWV in Asian populations. Therefore, in this study, we performed a two-stage GWAS to identify common loci using an Axiom™ Genome-Wide ASI 1 Array Plate with cardiovascular patients in Korea. Furthermore, to confirm the validity of our findings, we tested the association of the identified loci with PWV in a community-based cohort.
Methods
The study population consisted of 402 patients with cardiovascular disease diagnosed and treated at the Yonsei Cardiovascular Hospital as part of the Yonsei cardiovascular genome center cohort.
In the replication study, 1206 age- and gender-matched subjects were selected from the health examinees recruited in a community-based cohort in Mapo-gu, Seoul, Republic of Korea. All subjects were questioned about their clinical history, which included the assessment of traditional demographic information and cardiovascular risk factors (e.g. blood pressure and cholesterol fractions) with standard methods, as well as the assessment of arterial stiffness by PWV. Blood draws yielded lymphocytes for subsequent DNA extraction.
This study was approved by the local institutional review board, and all subjects, or their representatives, gave written informed consent (IRB no. 4-2001-0039 and no. 4-2011-0586). Body weight and height were measured without shoes and in light clothing, and body mass index was calculated. The blood pressure was measured with the dominant arm after being seated for 5 min, using an Omron HEM-780 (Omron Healthcare Co., Kyoto, Japan). The blood pressure was measured twice at 5 min intervals and the average value used for analysis. The blood pressure was measured before the measurement of PWV. PWV was determined by measuring the brachial–ankle pulse-wave velocity (baPWV) with a VP-2000 pulse-wave unit (Nippon Colin, Komaki City, Japan) for the GWAS and a VP-1000 pulse-wave unit (Omron Healthcare Co., Kyoto, Japan) for replication, as described previously (Citation12).
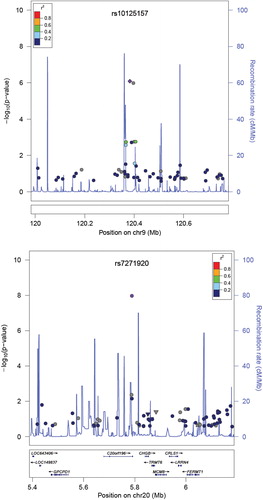
A GWAS was carried out on a single platform using the Axiom Genome-Wide ASI 1 Array Plate (Affymetrix, Inc., Santa Clara, CA, USA). In total, 402 samples were genotyped using 500 ng of genomic DNA and analyzed on a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) for purity, yield and concentration, and separated on a 1% agarose gel for integrity. After exclusion for the quality control procedure, in total, 502,731 single-nucleotide polymorphisms (SNPs) in the Axiom Genome-Wide ASI 1 Array were used for the final association analyses. In replication, variants that reached a p value of additive model < 9.0 × 10− 7 and clear cluster plot were selected. Genomic DNA was extracted from 5 ml peripheral blood samples by QuickGene SNP Kit DNA (Fujifilm, Tokyo, Japan). Taqman genotyping was performed using ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA). To ensure adequate control of type I error rates, log transformation was applied to PWV before analysis, to reduce the impact of outliers and minimize deviations from normality. Baseline clinical characteristics of the study subjects are presented as the mean ± SD for continuous variables and frequencies for discrete variables. To perform the genome-wide association analysis and replication study, a simple regression model was applied using the PLINK (http://pngu.mgh.harvard.edu/~purcell/plink) and SAS program (V. 9.1; SAS Institute Inc, Cary, NC, USA). The association was carried out using an additive genetic model and was adjusted for age, gender, systolic blood pressure and diastolic blood pressure. Regional plots were drawn using LocusZoom standalone version 1.1 based on Hap-Map Phase II JPT + CHB ().
Results
The demographic and clinical characteristics of the study cohort are shown in . The mean age was 59.5 ± 8.0 years (male: 59.2%), 64.2% had a history of hypertension, 17.4% had diabetes and 60.2% had previous coronary artery disease, and the mean baPWV was 1460.4 ± 299.2 cm/s in the GWAS set. The prevalence of hypertension and coronary artery disease and the use of antihypertensive medication were significantly higher in the GWAS set than in the replication cohort (). We tested the 502,731 SNPs for associations reaching nominal GWAS significance. The strongest and most significant association was observed in C20ORF196 rs7271920 (effect size = –0.17 ± 0.03, p = 7.15 × 10–9), followed by rs10125157 (effect size = –0.06 ± 0.01, p = 8.25 × 10–7) in GWAS (). However, the association with arterial stiffness was not replicated for these two loci in the replication cohort (rs7271920; effect size = –0.01 ± 0.01, p = 3.51 × 10–1, rs10125157; effect size = –0.01 ± 0.01, p = 3.10 × 10–1).
Table I. Clinical characteristics of the study subjects.
Discussion
We conducted a GWAS in the cardiovascular cohort, and found that SNPs (rs7271920 and rs10125157) were significantly associated with baPWV. However, these two loci were not successfully replicated in an independent sample within the community-based cohort.
The findings from this study are supported by a previous GWAS performed on 644 participants using a 100K Affymetrix Gene-Chip array that could not identify novel genetic variants associated with arterial stiffness (Citation8). In that study, none of the associations with the various markers of arterial stiffness reached genome-wide significance.
There are several potential reasons for the failure of validation of the loci identified in our study. First, several risk variants could escape detection because of the limited statistical power caused by the small effect size of the initial result. As such, variants with more modest effects were missed in the discovery analysis and were not carried forward to the replication set. We could not exclude the possibility that the effect size of the original reports could be represented as an exaggerated effect size. Furthermore, the power in the discovery GWAS is less favorable for rare variants or genes involving recessive mechanisms. When the heritability of PWV is considered as 2% (Citation10), the number needed is over 1900 cases with 83% statistical power, using PowerGWAS/QT (Version 1.1), which is a statistical program developed for medical investigators to estimate the power of detecting SNPs with quantitative traits in GWAS (Citation13). Secondly, the interactions of environmental exposures (e.g. lifestyle and other effect modifiers) could have an effect on the penetrance of these alleles. Previous studies have shown that arterial stiffness is strongly influenced by factors such as age, blood pressure, dyslipidemia, diabetes, renal function and inflammation (Citation14,Citation15). The results from this study suggest that the main determinants of arterial stiffness are exogenous risk factors rather than genetic risk factors. In addition, a limitation of this study is the inhomogeneity of the study population and the discrepancy in the prevalence of risk factors such as hypertension and coronary artery disease between the GWAS set and the replication set.
There are some merits of this study that need to be discussed. First, this was the first GWAS to determine genes associated with arterial stiffness in the Asian population. The discovery GWAS set was carried out in accurately phenotyped patients, and the replication study was performed in a relatively large sample of more than 1200 subjects. We used baPWV as a measurement of arterial stiffness as it is a relatively simple, non-invasive test that has been validated in terms of accuracy as well as being an independent predictor of cardiovascular events in the Asian population (Citation16–20). Future studies should consider multiple contributing factors when conceiving designs for GWAS.
Funding
This research was financially supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea [HI13C0715].
Declaration of interest: The authors report no conflicts of interest.
References
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
- Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–83.
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63.
- Matsuoka O, Otsuka K, Murakami S, Hotta N, Yamanaka G, Kubo Y, et al. Arterial stiffness independently predicts cardiovascular events in an elderly community – Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother. 2005;59(Suppl 1):S40–4.
- Mitsutake R, Miura S, Saku K. Association between coronary artery calcification score as assessed by multi-detector row computed tomography and upstroke time of pulse wave. Intern Med. 2007;46:1833–1836.
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390.
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670.
- Mitchell GF, DeStefano AL, Larson MG, Benjamin EJ, Chen MH, Vasan RS, et al. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation. 2005;112:194–199.
- Sayed-Tabatabaei FA, van Rijn MJ, Schut AF, Aulchenko YS, Croes EA, Zillikens MC, et al. Heritability of the function and structure of the arterial wall: findings of the Erasmus Rucphen Family (ERF) study. Stroke. 2005;36:2351–6.
- Levy D, Larson MG, Benjamin EJ, Newton-Cheh C, Wang TJ, Hwang SJ, et al. Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet. 2007;8(Suppl 1):S3.
- Tarasov KV, Sanna S, Scuteri A, Strait JB, Orru M, Parsa A, et al. COL4A1 is associated with arterial stiffness by genome-wide association scan. Circ Cardiovasc Genet. 2009; 2:151–8.
- Youn JC, Kim C, Park S, Lee SH, Kang SM, Choi D, et al. Adiponectin and progression of arterial stiffness in hypertensive patients. Int J Cardiol. 2013;163:316–9.
- Feng S, Wang S, Chen CC, Lan L. GWAPower: a statistical power calculation software for genome-wide association studies with quantitative traits. BMC Genet. 2011;12:12.
- Lee SJ, Park SH. Arterial ageing. Korean Circ J. 2013;43:73–9.
- Liao J, Farmer J. Arterial stiffness as a risk factor for coronary artery disease. Curr Atheroscler Rep. 2014;16:387.
- Kim HJ, Nam JS, Park JS, Cho M, Kim CS, Ahn CW, et al. Usefulness of brachial–ankle pulse wave velocity as a predictive marker of multiple coronary artery occlusive disease in Korean type 2 diabetes patients. Diabetes Res Clin Pract. 2009;85:30–34.
- Munakata M, Konno S, Miura Y, Yoshinaga K, J-TOPP Study Group. Prognostic significance of the brachial–ankle pulse wave velocity in patients with essential hypertension: final results of the J-TOPP study. Hypertens Res. 2012;35: 839–842.
- Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid–femoral and brachial–ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027.
- Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, et al. Brachial–ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–622.
- Yu WC, Chuang SY, Lin YP, Chen CH. Brachial–ankle vs carotid–femoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens. 2008;22:24–31.