Abstract
This paper presents a review of experimental and clinical research on the contribution of hypertension to cochlear hearing loss. Hypertension is one of the crucial risk factors underlying pathophysiological processes taking place in the cochlea. Several mechanisms explaining these processes have been described, mainly in animal models, such as the disturbance of the inner ear potassium recycling process due to the detrimental action of natriuretic hormone, and the decrease in the cochlear oxygen partial pressure. Current evidence linking hypertension to sensorineural high-frequency cochlear hearing loss in humans may be confounded by other concomitant diseases or risk factors such as age, coronary artery disease, diabetes, obesity, hyperlipidemia, smoking and noise exposure. Therefore, further research in this field is clearly needed.
Introduction
Hypertension is one the most common chronic diseases in adults. In the year 2000, it was found in 26.4% of the world's adult population, and the percentage is predicted to rise to 29.2% by 2025. The incidence of hypertension, similarly to hearing deficit, increases with age (Citation1,Citation2). Impairment of cochlear function is mentioned among other complications of hypertension such as myocardial infarction, stroke, retinopathy and nephropathy (Citation3–5). It is assessed that there are 360 million people in the world with disabling hearing loss (5.3% of the world's population) (Citation6). In the USA there were 29 million people (16.1% of the adult population) with hearing loss in 2004. In 2006, this number was assessed to reach 36 million and the main risk factors mentioned were smoking, noise exposure and cardiovascular risk factors (Citation7,Citation8). In this paper, we review the most important experimental and clinical studies that have reported on the influence of hypertension on hearing loss in the past 50 years.
Clinical studies
Hearing loss and hypertension
A hypothesis on the detrimental effect of hypertension on cochlear and vestibular systems in humans was developed early in the twentieth century (Citation3,Citation9–11). Since then, many studies have been conducted, with contradictory results. In a study by Baraldi et al. (Citation12), conducted in elderly people, the degree of hearing loss was similar in the normotensive and the hypertensive groups; however, the audiometric configuration in the hypertensive group was different from the non-hypertensive group. Torre et al. (Citation13) evaluated the relationship between self-reported cardiovascular disease and cochlear function in 1501 older adults. Cochlear function was measured using distortion product otoacoustic emissions (OAEs). The only statistically significant correlation found was that women with a self-reported history of myocardial infarction were twice as likely to have cochlear impairment as women without such a history. No other cardiovascular risk factors were associated with cochlear impairment. On the other hand, de Moraes Marchiori et al. (Citation14) studied pure-tone audiometry results and blood pressure measurements in middle-aged people (45–64 years of age). In a group of subjects with hearing loss, 46.8% had hypertension, which was found in only 29.9% of subjects with normal hearing. The type of hearing loss (mild sensorineural) was similar in both groups. Regression analysis showed arterial hypertension, advanced age and male gender to be independent risk factors for the hearing loss. Similarly, Agarwal et al. (Citation15) compared the hearing of 150 hypertensive patients with that of 124 healthy volunteers. They concluded that hypertensive patients with blood pressure over 180/110 mmHg had worse hearing thresholds in high frequencies. Similar findings were obtained by Gates et al. (Citation16). Moreover, they found a correlation between hypertension and low-frequency hearing loss in women. Tan et al. (Citation17) studied the influence of hypertension on hearing in patients with hypertensive retinopathy. They confirmed statistically significant impairment of hearing thresholds in hypertensive patients for 2, 4 and 8 kHz frequencies in comparison with the control group. Furthermore, they found worse results for the hearing threshold for 4 and 8 kHz frequencies in patients with grade I retinopathy in comparison with hypertensive patients without retinal pathology and with a control group. The authors suggested that it is the hypertensive microangiopathy in the cochlear and retinal vessels that causes hearing and eyesight loss, respectively. Similarly, Esparza et al. (Citation18) compared inner ear and retinal function in hypertensive patients (29–64 years of age) and in healthy subjects. Hypertensive subjects had worse hearing for 8 kHz and worse evoked OAE results. Over 60% of the hypertensive patients had retinopathy that correlated positively with worse hearing threshold and with lack of response from the hair cells in OAE for frequencies of 4–8 kHz. From these results, it seems reasonable to refer a hypertensive patient with retinal angiopathy for audiometric tests. Results of the clinical studies on hearing loss due to hypertension alone are inconsistent, although it seems significant that high-frequency sensorineural hearing loss is predictable among patients with hypertension.
Impact of other cardiovascular risk factors
Hypertension often coexists with other risk factors such as type 2 diabetes and dyslipidemia. These multifactorial inheritance chronic disorders (MICDs) develop through the interaction of environmental and epigenetic factors. Moreover, each of the diseases increases the risk of the other conditions, and their effect on target organs seems to be synergistic. The effect of MICDs on cochlear and vestibular dysfunction was assessed by Chávez-Delgado et al. (Citation19), who studied a group of 385 patients with MICDs with use of the tone audiometry and electronystagmography. Hearing loss was as common in the group of hypertensive patients as in the group with hypertension, type 2 diabetes and dyslipidemia. It was also found that cochlear dysfunction was more common than cochleovestibular impairment in all of the MICD patients.
Chen and Ding (Citation20) found that hypertension together with hyperlipidemia worsens the hearing of elderly people. Friedland et al. (Citation21) planned to confirm the correlation between cardiovascular risk factors and hearing loss in low frequencies by means of tone audiometry. A group of 1168 smoking patients with hypertension, diabetes and hyperlipidemia was studied. In this study, slope type hearing loss, which begins in 0.5–2 kHz frequencies and is more profound in high frequencies, was inadequately reported as low-frequency hearing loss. This can be misleading since most scientists associate these comorbidities with high-frequency hearing loss. In multivariate regression analysis, hypertension correlated with the above-mentioned hearing loss and with another type of audiometric configuration called “strial”, i.e. ≥ 25 dB hearing loss between 0.5 and 2 kHz with ≤ 15 dB variability, combined with disturbance of the stria vascularis. The authors recommended that patients with these two types of audiogram should be followed up for cardiovascular risk factors.
Agrawal et al. (Citation7) assessed the effect of microvascular risk factors on hearing in a large group of 3853 American subjects. Hypertension was found in 27% of the subjects and turned out to be a weak risk factor, and hearing loss was found only in the range around 1 kHz. A larger population of 26,917 men (40–74 years of age) including 3488 cases of hearing loss was studied by Shargorodsky et al. (Citation22), who did not find statistically significant correlations between hypertension, diabetes, obesity and hearing impairment. In this study, the multivariate regression analysis proved statistically significant only hypercholesterolemia and smoking. In another study, hearing loss was assessed in 12 patients with hypertension and diabetes (average blood pressure 146/78 mmHg) and in 10 normotensive patients with diabetes (Citation23). All patients were tested with tonal audiometry including air and bone conduction, speech reception threshold and speech discrimination score. In each frequency (0.25–8 kHz), there was a significant increase in the hearing loss between the normotensive and the hypertensive insulin-dependent diabetics. However, patients with hypertension and diabetes were 15 years older than those with normal blood pressure. When age was taken into consideration and the results were re-evaluated, a weak, although statistically significant, difference was found only in frequencies of 4–8 kHz (p = 0.048). The average speech reception threshold of the normotensive insulin-dependent diabetics was 11 ± 2.69 dB and that of the hypertensive insulin-dependent diabetics was 26.67 ± 4.48 dB (Citation23).
In people above 60 years of age, hearing loss is often accompanied by tinnitus. Sometimes it is the tinnitus that prompts patients with unrecognized hypertension to visit their general practitioner or otolaryngologist. Gibrin et al. (Citation24) studied tinnitus in patients with hypertension and diabetes, and found that only the comorbidity of diabetes mellitus and hypertension was an independent risk factor for tinnitus. In hypertensive patients with other cardiovascular risk factors such as diabetes and hyperlipidemia, high-frequency sensorineural hearing loss spreads also to medium and low frequencies. This has a crucial influence on understanding speech.
Hearing loss and age
Age is the most important risk factor for hearing loss. Many authors emphasize that presbyacusis has the same audiometric characteristics as hearing loss due to hypertension: high-frequency bilateral and symmetrical sensorineural hearing loss. Some authors introduce corrections on age in their analyses of hearing loss; however, changes caused by aging can be individually different (Citation25–29). Thus, it is very difficult to discriminate the hearing loss component dependent on hypertension in elderly people. However, certain studies have shown that cochlear dysfunction is too profound to be caused only by aging in hypertensive patients (Citation18,Citation19). In addition, in another study of elderly people, low cardiovascular fitness was correlated with lower hearing threshold in frequencies of 2 kHz and 4 kHz (Citation30). In a study on patients with ischemic stroke, the pathophysiology of which is strictly related to vascular risk factors such as hypertension, age above 60 years was a crucial risk factor for sensorineural hearing loss (Citation5).
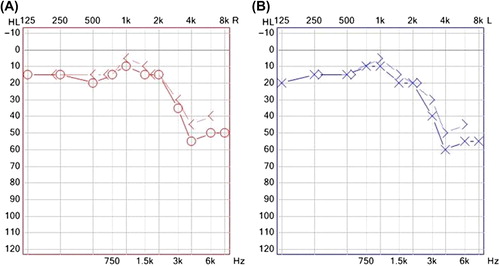
Cochlear presbyacusis is symmetrical high- frequency sensorineural hearing loss caused by physiological aging of the cochlea. Hypertension seems to augment this process, as illustrated in , which presents an audiogram of a 45-year-old patient with hypertension.
Hearing loss and noise
Since a multifactorial etiology of hearing loss seems to be most probable, many studies have been conducted to determine the possible correlations and/or additive effects of hypertension and noise. Transient noise exposure causes reversible changes in the vascular system and in the cochlea, and chronic exposure intensifies these changes significantly (Citation31–34). It has also been found that deaf–mute children have lower blood pressure than their normal hearing age-mates (Citation35). Multiple regression analysis revealed that body mass index, age and hearing ability explained a significant amount of the variation in blood pressure range in these children. On the basis of these results, it was suggested that noise exposure is associated with higher systolic and diastolic blood pressure. A surprising finding of Malinow et al. (Citation36) was an increase in blood pressure in deaf people who sign. This study suggests independence of this communication method from vocalization, which has a substantial influence on the cardiovascular system.
The evidence linking chronic noise exposure to human hypertension (Citation16,Citation27,Citation29,Citation32,Citation37,Citation38) is consistent with previous reports from animal studies (Citation39,Citation40). However, Hirai et al. (Citation41), who conducted a study on a large group of 2124 factory workers, could not find a correlation between increased blood pressure and noise exposure at work. The authors explained that their observations could be due to adaptation of workers to chronic noise. They also suggested that stress caused by noise might induce hypertension only if a genetic predisposition for hypertension was present. Several other studies also failed to find an independent correlation between noise exposure and elevated blood pressure (Citation42–45). Results of a study by Zhao et al. (Citation46) in 1101 female workers in a textile mill in Beijing, China, indicated that cumulative exposure to noise, as measured by years worked in a particular noise environment, did not predict the risk of hypertension. Belli et al. (Citation47) studied hypertension in over 400 textile factory workers exposed to noise of over 100 dB and in workers not exposed to noise. No difference was found in the number of subjects with hypertension between the two groups; however, hypertension was more advanced in noise-exposed workers.
Other studies have investigated the combined effect of hypertension and noise on hearing loss. In a study by Ni et al. (Citation48), an increased risk of high-frequency hearing loss was found in noise-exposed women with low artery compliance. Tarter and Robins (Citation49) reported correlations between hypertension, 4 kHz hearing loss and long-term (minimum 5 years) work in noise of over 85 dB, only in African-Americans. This correlation was not found in Caucasian workers. Industrial and environmental noise can simultaneously cause damage to the organ of Corti and increase blood pressure through the mechanism of stress. In a study comparing long-distance and city bus drivers, hypertension was three times more common in the former group, and was associated with 0.25–2 kHz hearing loss, especially in the left ear. These differences were attributed to higher stress levels in the long-distance bus drivers, but the contribution of noise exposure on the side of the usually opened window of the driver's cab was not ruled out (Citation50).
Idiopathic sudden sensorineural hearing loss
Two of the authors of this review (TP and DG) proved in multivariate stepwise linear regression that hypertension is a crucial risk factor in sensorineural hearing loss in patients with ischemic cerebral stroke (Citation5). However, this seems not to be true for idiopathic sudden sensorineural hearing loss (ISSHL), as studied by Chang et al. (Citation51). In that study, ISSHL was not associated with an increased stroke risk, although both disorders have similar vascular etiology. In another study, ISSHL was statistically more common in patients with diabetes and hypercholesterolemia; however, hypertension alone was not linked to an increased risk of this type of hearing loss (Citation52). Duck et al. (Citation23) observed cerebral microangiopathy in patients with ISSHL and hypertension; however, these patients also had diabetes and hyperlipidemia and were of older age. Similarly, Nagaoka et al. (Citation53) found that ISSHL correlates with higher rates of cerebral microangiopathy (85%) and slower improvement of speech understanding in older patients with hypertension, diabetes and hyper- lipidemia. Hirano et al. (Citation54) found that the results of treatment of ISSHL in patients with coexisting microangiopathic diseases (hypertension, diabetes and hyperlipidemia) were worse than in patients without these comorbidities; however, the latter group was younger. In the study by Mosnier et al. (Citation55), hypertension, diabetes, hyperlipidemia and cigarette smoking were not related to the risk of ISSHL. While hypertension causes bilateral sensorineural hearing loss, ISSHL is commonly connected with unilateral sensorineural hearing loss. However, there are some single reports on ISSHL observed after a sudden reduction in blood pressure (Citation56,Citation57). The possible cause of hearing loss in such cases could be an asymmetrical cochlear ischemia. Chao (Citation56) reported a case of a 46-year-old woman with malignant hypertension who presented “cookie bite” low-frequency hearing loss when her blood pressure was lowered from 237/144 mmHg to 155/85 mmHg with an infusion of sodium nitroprusside.
Animal studies
In the early 1960s, a new strain of genetically modified rats with many similarities to humans was created, named spontaneously hypertensive (SH) rats (Citation58,Citation59). In the 1980s, Borg (Citation60) documented a deterioration in high-frequency hearing sensitivity in older SH rats in comparison with normotensive animals. Because of the physiology of the rodents’ hearing and the difference in frequencies of hearing tested, direct transposition of the results to humans is difficult. However, Borg (Citation61) showed that exposure to high-frequency noise of 100 dB causes much greater loss of cochlear hair cells in SH rats than in normotensive ones. Moreover, he compared the influence of noise on SH rats with rats with hypertension induced by renal artery occlusion and with a control group (Citation62). SH rats had significantly larger hearing loss in comparison with other strains. The duration of induced hypertension was too short to cause substantial vascular damage in rats with renal artery occlusion. Axelsson et al. (Citation63) also studied noise exposure in SH rats. It was reported that 100 dB noise lasting for 10 h caused constriction of precapillary sphincters in radial arterioles and thus a reduction in blood flow. This phenomenon took place in both hypertensive and normotensive animals, but was more pronounced in the hypertensive group. One possible explanation given for this difference was vascular damage due to the preceding presence of vascular factors such as hypertension. Pillsbury (Citation64) studied the synergistic influence of hypertension, atherogenic diet and noise exposure on hearing in rats. Hypertension and atherogenic diet alone did not cause substantial hearing loss of 4, 8 and 20 kHz frequencies. However, SH rats that were exposed to 95 dB noise and were fed an atherogenic diet had worse hearing than SH rats exposed to noise but which received a normal diet. Thus, it was concluded that diet does not cause hearing loss alone, but it causes cochlear damage when associated with hypertension and chronic noise exposure. The additive effect of these various factors is supported by studies linking noise to blood pressure increase in rats (Citation39,Citation40). Thus, the influence of hypertension on the inner ear causes the cochlea to be more vulnerable to noise. Atherogenic diet seems to augment this detrimental effect.
The relationship between hypertension and hearing loss may be affected by glucose metabolism. Duck et al. (Citation23) studied the detrimental effects on hearing of diabetes and hypertension. No significant differences were found in the loss of hair cells between diabetic rats with and without hypertension.
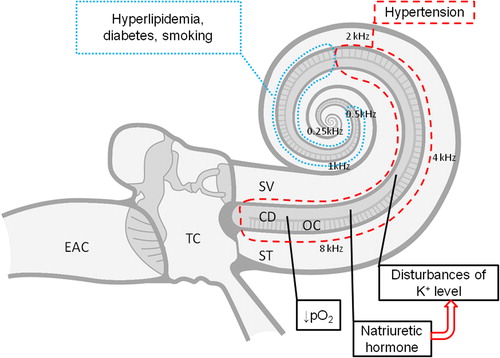
The mechanisms underlying the relationship between cochlear damage and hypertension are poorly understood. In 1984, Tachibana et al. (Citation65) suggested that the primary target of hypertensive damage in the rat cochlea is the vascular stria feeding the organ of Corti. Oxygen diffuses to the organ of Corti through the endolymph from the vascular stria and from the middle ear through the round and oval windows (Citation66–68). Normally, the partial pressure of oxygen (pO2) differs in various parts of the cochlear duct, but it is always lowest near the organ of Corti (Citation67–71). Thus, even a small decrease in pO2 can cause cochlear damage.
McCormick et al. (Citation72) reported a positive correlation between cochlear potentials in hypertensive rats and systolic blood pressure. Similarly to Tachibana et al. (Citation65), they postulated that natriuretic hormones may accelerate the development of sensorineural hearing loss by inhibiting the potassium pump in the cochlear stria vascularis. Flexion of the stereocilia of the hair cells causes opening of K+ canals and an influx of potassium from the endolymph to the hair cells. The influx causes depolarization and activation of calcium canals, which in turn causes outflow of K+ ions, resulting in repolarization. Extracellular K+ ions are then transported through junctions between the supporting cells to the stria vascularis and then back to the endolymph (ionic K+ recycling). Inhibiting the potassium pump in the stria vascularis stops the K+ influx to the hair cells and thus stops their depolarization (Citation73). The disturbance of the concentration of ions, mainly K+ in the endolymph and in the hair cells, was observed in an SH rat model of age-related hearing loss in the study by Rarey et al. (Citation74).
Taken together, previous studies indicate that malfunction of the stria vascularis seems to be the most important factor in hypertension-related cochlear damage. Subclinical damage to the vascular stria includes the decrease in the cochlear oxygen partial pressure and disturbance of the ionic K+ recycling.
Summary
The results of the clinical studies, although not unequivocal, indicate that hypertension may be an important risk factor in sensorineural high-frequency hearing loss. This link is affected by confounding factors such as diabetes, noise exposure, stress, advanced age and gender. Hearing loss in hypertensive patients with comorbidities, besides high tones, also affects middle and low tones. These complex relationships, including potential underlying mechanisms, are summarized in . It should be noted that our current pathophysiological knowledge comes primarily from animal studies. Clearly, we need more human studies based on novel methods assessing both hearing loss and cardiovascular function and structure. These studies should clarify the current doubts on whether hearing loss should be listed among other forms of hypertension-related target organ damage, such as left ventricular hypertrophy, increased carotid intima–media thickness or brain white matter lesions. Better understanding of the relationship between high-frequency hearing loss and hypertension may have important clinical implications. Early referral of patients with hypertension to audiometric testing may become one of the early disability prevention strategies.
Figure 2. Pathophysiological mechanisms of hypertensive high-tone sensorineural hearing loss. Black dashed line: part of the cochlea affected in arterial hypertension; black dotted line: part of the cochlea additionally affected in the presence of other comorbidities (hyperlipidemia, diabetes) or addictions (smoking). EAC, external auditory canal; TC, tympanic cavity; SV, scala vestibuli; ST, scala tympani; CD, cochlear duct; OC, organ of Corti; K+, potassium ion concentration; pO2, partial pressure of oxygen.
Declaration of interest: The authors report no conflicts of interest.
References
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
- Pereira M, Carreira H, Vales C, Rocha V, Azevedo A, Lunet N. Trends in hypertension prevalence (1990–2005) and mean blood pressure (1975–2005) in Portugal: a systematic review. Blood Press. 2012;21:220–6.
- Hansen CC, Denmark A. Perceptive hearing loss and arterial hypertension. Arch Otolaryngol. 1968;87:119–22.
- Oparil S, Kjeldsen SE, Narkiewicz K, Hedner T. Blood pressure variability: emerging role in risk assessment and therapeutics. Blood Press. 2010;19:209–11.
- Przewoźny T, Gąsecki D, Narożny W, Nyka W. Risk factors of sensorineural hearing loss in patients with ischemic stroke. Otol Neurotol. 2008;29:745–50.
- World Health Organization. WHO global estimates on prevalence of hearing loss. Mortality and burden of diseases and prevention of blindness and deafness. WHO; 2012. Available from http://www.who.int/pbd/deafness/WHO_GE_HL.pdf.
- Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med.2008;168: 1522–30.
- Pleis JR, Lethbridge-Cejku M. Summary health statistics for US adults: National Health Interview Survey, 2006, Vital Health Stat 10. 2007;235:1–153.
- Bunch CC. Further observations on age variations in auditory acuity. Arch Otolaryngol. 1931;13:170–80.
- Rosen S, Bergman M, Plester D, El-Mofty A, Satti MH. Presbyacusis study of a relatively noise-free population in Sudan. Ann Otol. 1962;71:727.
- Rosen S, Plester D, El-Mofty A, Rosen HV. Relation of hearing loss to cardiovascular disease. Trans Am Acad Ophthalmol Otolaryngol. 1964;68:433–44.
- Baraldi GS, Almeida LC, Borgea ACLC. Hearing loss and hypertension: findings in an older by group. Rev Bras Otorrinolaringol. 2004;70:640–4.
- Torre P III, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. The association between cardiovascular disease and cochlear function in older adults. J Speech Lang Hear Res. 2005;48:473–81.
- de Moraes Marchiori LL, de Almeida Rego Filho E, Matsuo T. Hypertension as a factor associated with hearing loss. Braz J Otorhinolaryngol. 2006;72:533–40.
- Agarwal S, Mishra A, Jagade M, Kasbekar V, Nagle SK. Effects of hypertension on hearing. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 3):614–8.
- Gates GA, Cobb JL, D’Agostinho RB, Wolf A. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk Factors. Arch Otolaryngol Head Neck Surg. 1993;119:156–61.
- Tan TY, Rahmat O, Prepageran N, Fauzi A, Norah NH, Raman R. Hypertensive retinopathy and sensorineural hearing loss. Indian J Otolaryngol Head Neck Surg. 2009;61:275–9.
- Esparza CM, Jáuregui-Renaud K, Morelos CM, Muhl GE, Mendez MN, Carillo NS, et al. Systemic high blood pressure and inner ear dysfunction: a preliminary study. Clin Otolaryngol. 2007;32:173–8.
- Chávez-Delgado ME, Vázquez-Granados I, Rosales-Cortés M, Velasco-Rodríguez V. Cochleovestibular dysfunction in patients with diabetes mellitus, hypertension and dyslipidemia. Acta Otorrinolaringol Esp. 2012;63:93–101.
- Chen YL, Ding YP. Relationship between hypertension and hearing disorders in the elderly. East Afr Med J. 1999;76: 344–7.
- Friedland DR, Cederberg C, Tarima S. Audiometric pattern as a predictor of cardiovascular status: development of a model for assessment of risk. Laryngoscope. 2009;119:473–86.
- Shargorodsky J, Curhan SG, Eavey R, Curhan GC. A prospective study of cardiovascular risk factors and incident hearing loss in men. Laryngoscope. 2010;120:1887–91.
- Duck SW, Prazma J, Bennett PS, Pillsbury HC. Interaction between hypertension and diabetes mellitus in the pathogenesis of sensorineural hearing loss. Laryngoscope. 1997;107: 1596–605.
- Gibrin PC, Melo JJ, Marchiori LL. Prevalence of tinnitus complaints and probable association with hearing loss, diabetes mellitus and hypertension in elderly. Codas. 2013;25: 176–80.
- Brant LJ, Gordon-Salant S, Pearson JD, Klein LL, Morrell CH, Metter EJ, et al. Risk factors related to age-associated hearing loss in the speech frequencies. J Am Acad Audiol. 1996;7: 152–60.
- Lee KY. Pathophysiology of age-related hearing loss (peripheral and central). Korean J Audiol. 2013;17:45–9.
- Leithäuser D. Hearing in old age. Hearing loss is never physiologic. MMW Fortschr Med. 1999;141:36–8.
- Linssen AM, van Boxtel MP, Joore MA, Anteunis LJ. Predictors of hearing acuity: cross-sectional and longitudinal analysis. J Gerontol A Biol Sci Med Sci. 2014;69:759–65.
- Makishima K. Arteriolar sclerosis as a cause of presbycusis. Otolaryngology. 1978;86:322–6.
- Hutchinson KM, Alessio H, Baiduc RR. Association between cardiovascular health and hearing function: pure-tone and distorsion product otoacoustic emission measures. Am J Audiol. 2010;19:26–35.
- Cantrell RW. Prolonged exposure to intermittent noise: audiometric, biochemical, motor, psychological and steep effects. Laryngoscope. 1974;84(Suppl 1):1–55.
- Jonsson A, Hansson L. Prolonged exposure to a stressful stimulus (noise) as a cause of raised blood pressure in man. Lancet. 1977;i:86–7.
- Mikovic-Kraus S. Noise induced hearing loss and blood pressure. Int Arch Occup Environ Health. 1990;62: 259–60.
- Narlawar UW, Surjuse BG, Thakre SS. Hypertension and hearing impairment in workers of iron and steel industry. Indian J Physiol Pharmacol. 2006;50:60–6.
- Wu TN, Chiang HC, Huang JT, Chang PY. Comparison of blood pressure in deaf–mute children and children with normal hearing: association between noise and blood pressure. Int Arch Occup Environ Health. 1993;65:119–23.
- Malinow KL, Lynch JJ, Foreman PJ, Friedmann E, Thomas SA. Blood pressure increases while signing in a deaf population. Psychosom Med. 1986;48:95–101.
- Maininen O, Aro S. Noise-induced hearing loss and blood pressure. Int Arch Occup Environ Health. 1972;42:251–6.
- Saad MM, Hussein MS, Hammam HM. Study of noise, hearing impairment and hypertension in Egypt. Ann Saudi Med. 1994;14:307–11.
- Hallbäck M, Folkow B. Cardiovascular response to acute mental stress in spontaneously hypertensive rats. Acta Physiol Scand. 1974;90:684–98.
- Peterson EA, Augenstein JS, Tanis DC, Augenstein DG. Noise raises blood pressure without impairing auditory sensitivity. Science. 1981;211:1450–2.
- Hirai A, Takata M, Mikawa M, Yasumoto K, Iida H, Sasayama S, Kagamimori S. Prolonged exposure to industrial noise causes hearing loss but not high blood pressure: a study of 2124 factory laborers in Japan. J Hypertens. 1991;9: 1069–73.
- Hedstrand H, Drettner B, Klockhoff I, Svedberg A. Noise and blood-pressure. Lancet. 1977;ii:1291.
- Takala J, Varke S, Vaheri E, Sievers K. Noise and blood-pressure. Lancet. 1977;ii:974–5.
- Wu TN, Chou FS, Chang PY. A study of noise-induced hearing loss and blood pressure in steel mill workers. Int Arch Occup Environ Health. 1987;59:529–36.
- Ylikoski ME. Self-reported elevated blood pressure in army officers with hearing loss and gunfire noise exposure. Mil Med. 1995;160:388–90.
- Zhao YM, Zhang SZ, Selvin S, Spear RC. A dose response relation for noise induced hypertension. Br J Ind Med. 1991;48:179–84.
- Belli S, Saini L, Scarficcia G, Sorrentino R. Arterial hypertension and noise. A cross-sectional study. Am J Ind Med. 1984;6:59–65.
- Ni CH, Chen ZY, Zhou Y, Zhou JW, Pan JJ, Liu N, et al. Associations of blood pressure and arterial compliance with occupational noise exposure in female workers of textile mill. Chin Med J (Engl). 2007;120:1309–13.
- Tarter SK, Robins TG. Chronic noise exposure, high-frequency hearing loss, and hypertension among automotive assembly workers. J Occup Med. 1990;32:685–9.
- Abdelmoneim I. Hearing impairment and hypertension among long distance bus drivers. J Family Community Med. 2003;10:25–9.
- Chang CF, Kuo YL, Chen SP, Wang MC, Liao WH, Tu TY, Shiao AS. Relationship between idiopathic sudden sensorineural hearing loss and subsequent stroke. Laryngoscope. 2013;123:1011–5.
- Aimoni C, Bianchini C, Borin M, Ciorba A, Fellin R, Martini A, et al. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: a case–control study. Audiol Neurootol. 2010;15:111–5.
- Nagaoka J, Anjos MF, Takata TT, Chaim RM, Barros F, Penido Nde O. Idiopathic sudden sensorineural hearing loss: evolution in the presence of hypertension, diabetes mellitus and dyslipidemias. Braz J Otorhinolaryngol. 2010;76: 363–9.
- Hirano K, Ikeda K, Kawase T, Oshima T, Kekehata S, Takahashi S, et al. Prognosis of sudden deafness with special reference to risk factors of microvascular pathology. Auris Nasus Larynx. 1999;26:111–5.
- Mosnier I, Stepanian A, Baron G, Bodenez C, Robier A, Meyer B, et al. Cardiovascular and thromboembolic risk factors in idiopathic sudden sensorineural hearing loss: a case–control study. Audiol Neurootol. 2011;16:55–66.
- Chao TK. Sudden sensorineural hearing loss after rapid reduction of blood pressure in malignant hypertension. Ann Otol Rhinol Laryngol. 2004;113:73–5.
- Ross UH, Brademann G, Lehnhardt E.[Acute hearing loss caused by arterial hypotension]. HNO. 1993;41:436–9.
- Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–93.
- Trippodo NC, Frohlich ED. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ Res. 1981;48: 309–19.
- Borg E. Auditory thresholds in rats of different age and strain. A behavioral and electrophysiological study. Hear Res. 1982; 8:101–15.
- Borg E. Noise-induced hearing loss in normotensive and spontaneously hypertensive rats. Hear Res. 1982;8:117–30.
- Borg E. Noise-induced hearing loss in rats with renal hypertension. Hear Res. 1982;8:92–3.
- Axelsson A, Borg E, Hornstrand C. Noise effects on the cochlear vasculature in normotensive and spontaneously hypertensive rats. Acta Otolaryngol. 1983;96:215–25.
- Pillsbury HC. Hypertension, hyperlipoproteinemia, chronic noise exposure: is there synergism in cochlear pathology? Laryngoscope. 1986;96:1112–38.
- Tachibana M, Yamamichi I, Nakae S, Hirasugi Y, Machino M, Mizukoshi O. The site of involvement of hypertension within the cochlea. A comparative study of normotensive and spontaneously hypertensive rats. Acta Otolaryngol. 1984; 97:257–65.
- Maass B, Baumgärtl H, Lübbers DW. Lokale pO2- und pH2-Messungen mit Nadelelektroden zum Studium der Sauerstoffürsorgung und Mikrozirkulation des Innenohres. Arch Otorhinolaryngol. 1976;214:109–24.
- Morgenstern C, Kessler M. Oxygen consumption and oxygen distribution in the inner ear. Arch Otorhinolaryngol. 1978;220:159–62.
- Tanaka K, Motomura S. Permeability of the labyrinthine windows in guinea pigs. Arch Otorhinolaryngol. 1981;233: 67–75.
- Misrahy FA, Shinabarger EW, Arnold JE. Changes in cochlear endolymphatic oxygen availability, action potential and microphonics during and following asphyxia, hypoxia and exposure to loud sounds. J Acoust Soc Am. 1958;30:701–4.
- Thorne PR, Nuttall AL. Alternations in oxygenations of cochlear endolymph during loud sound exposure. Acta Otolaryngol (Stockh). 1989;107:71–9.
- Tsunoo M, Perlman HB. Cochlear oxygen tension. Relation to blood flow and function. Acta Otolaryngol (Stockh). 1964;590:437–50.
- McCormick JG, Harris DT, Hartley CB, Lassiter RB. Spontaneous genetic hypertension in the rat and its relationship to reduced ac cochlear potentials: implications for preservation of human hearing. Proc Natl Acad Sci U S A. 1982; 79:2668–72.
- Ikeda K, Morizono T. Electrochemical profiles for monovalent ions in the stria vascularis: cellular model of ion transport mechanisms. Hear Res. 1989;39:279–86.
- Rarey KE, Ma YL, Gerhardt KJ, Fregly MJ, Garg LC, Rybak LP. Correlative evidence of hypertension and altered cochlear microhomeostasis: electrophysiological changes in the spontaneously hypertensive rat. Hear Res. 1996;102: 63–9.