Abstract
Acetaminophen (APAP), also known as paracetamol, is the commonest cause of toxic ingestion in the world. Because overdose of APAP has life-threatening effects on kidney, treatment of APAP-induced nephrotoxicity has life-saving importance. Aim of the study was to evaluate the efficacy of medical ozone therapy in experimental model of APAP toxication. Twenty-one male Wistar rats (200–250 g) were randomly assigned into three groups containing seven rats each: Sham, control (only APAP treated), and APAP + ozone therapy groups. Rats were killed 48 hours after administration of APAP. Urea, creatinine levels in the blood, and malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activity in renal tissue were measured. Kidney tissues were stained with hematoxylin and eosin for histological assessment. APAP administration deteriorated the renal functions and significantly elevated renal MDA levels and depleted SOD and GSH-Px activities. Ozone therapy significantly reduced the MDA level, increased the SOD and GSH-Px activities, and normalized the renal histology. In conclusion, our study results are consistent with encouraging data for ozone therapy on APAP-induced nephrotoxicity in rats by improving antioxidant mechanism and oxidative stress.
INTRODUCTION
Acetaminophen (APAP), also known as paracetamol, is one of the most widely used analgesic and antipyretic drug and produces hepatocyte and renal tubular necrosis in humans and animals at overdoses. In children, APAP is the commonest cause of toxic ingestion in the world. Although APAP-induced liver damage has been studied extensively, in the kidney its toxicity has not been clearly understood. Renal insufficiency occurs in approximately 1–2% of the patients with an overdose of APAP.Citation1–4 The main toxicity of APAP is the result of drug metabolism in the liver and other organs. At the pharmacologic doses, APAP is metabolized mostly to inactive compounds via Phase II reactions by conjunction by sulfate and glucuronide. Because over dose of APAP has life-threatening effects on kidney, treatment of APAP-induced nephrotoxicity has life-saving importance.Citation4,Citation5
Although it is not clear, oxidative stress is reported to play an important role in the pathogenesis of APAP-induced renal damage, as evidenced by an increase in the lipid peroxidation and the depletion of intracellular glutathione (GSH).Citation4–6
A gas mixture comprising ozone/oxygen used in medicine is known as medical ozone therapy. It was demonstrated that ozone increases antioxidant enzyme activities such as glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase, preparing the host to face physiopathologic conditions mediated by reactive oxygen species (ROS),Citation7–9 as reported in our previous works.Citation7,Citation10 Clinically important beneficial effects of ozone therapy in some disorders such as peritonitis, infected wounds, skin ulcers, and gangrenes have been reported.Citation8,Citation9 In this study we hypothesized that the ozone therapy may have protective effects in rats with APAP-induced nephrotoxicity by improving antioxidant activity. For that reason we designed a study to evaluate the efficacy of medical ozone therapy in experimental model of APAP toxicities.
MATERIAL AND METHODS
Animals and study groups
All animal procedures were approved by the institutional committee on the care and use of animals of our institution. Twenty-one male Wistar rats (200–250 g) provided by the animal laboratory of our institute were randomly assigned into three groups containing seven rats each: sham, control (A) (only APAP treated), and ozone (APAP + ozone therapy) (A + OT) groups. Before the experiment, the animals were fed standard rat chow and water ad libitum and housed in cages with controlled temperature and 12-hour light/dark cycle for at least 1 week.
Induction of acetaminophen toxicity
A suspension of APAP (1.0 g/3 mL/kg) (Army Drug Factory, Ankara, Turkey) was made in hot distilled water and administered orally according to Chattopadhyay's studyCitation11 and administrated to rats in control and ozone groups at dose of 1 g/kg via an oral gavage. The animals were returned to their cages to recover. Water and food were available ad libitum.
Treatment modality
Immediately after the administration of single-dose APAP, the rats in the A + OT group were administered the ozone/oxygen mixture at a dosage of 0.7 mg/kg via the intraperitoneal route for single dose. Ozone (O3) was generated by the ozone generator (Ozonosan Photonik 1014; Hansler GmbH, Iffezheim, Germany), allowing control of the gas flow rate and ozone concentration in real time by a built-in UV spectrometer. The ozone flow rate was kept constant at 3 L/min, representing a concentration of 60 mg/mL and a gas mixture of 97% O2 + 3% O3. Tygon polymer tubes and single-use silicon-treated polypropylene syringes (ozone resistant) were used throughout the reaction to ensure containment of O3 and consistency of concentrations.
Biochemical analysis
The harvested renal tissue samples were stored at −80°C to study antioxidant enzyme activity and tissue lipid peroxidation. The frozen tissues were homogenized in phosphate buffer (pH 7.4) by means of homogenization (Heidolph Diax 900; Heidolph Elektro GmbH, Kelhaim, Germany) on an ice cube. The supernatant was used for the entire assay. Initially, the protein content of the tissue homogenates was measured by the method of Lowry et al.,Citation12 with bovine serum albumin used as the standard for all assays. Lipid peroxidation level was measured with the thiobarbituric acid reaction by the method of Ohkawa et al.Citation13 This method was used to obtain a spectrophotometric measurement of the color produced during the reaction of thiobarbituric acid with malondialdehyde (MDA) at 535 nm. The calculated MDA levels were expressed as millimoles per gram protein. SOD activity was assayed using the nitroblue tetrazolium (NBT) by the method of Durak et al.Citation14 In this method, NBT was reduced to blue formazan by, which has a strong absorbance at 560 nm. One unit of SOD is defined as the amount of protein that inhibits the rate of NBT reduction by 50%. The estimated SOD activity was expressed as units per gram protein. The GSH-Px activity was measured using the method described by Paglia and Valentine,Citation15 in which GSH-Px activity was coupled with the oxidation of nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) by GSH reductase. The oxidation of NADPH was spectrophotometrically followed up at 340 nm at 37°C. The absorbance at 340 nm was recorded for 5 min. The activity was the slope of the lines expressed as millimoles of NADPH oxidized per minute. GSH-Px activity was presented as units per gram protein. Serum urea and creatinine levels were measured with a spectrophotometric technique by the Olympus AU-2700 autoanalyzer using commercial kits (Olympus, Hamburg, Germany) and presented as units per liter.
Histopathological examination
All the surviving animals were killed on the 48 hours of the experiment via decapitation, the abdomen was opened, and both kidneys of each animal were taken for histopathologic evaluation. Tissue blocks for light microscopy were fixed in 10% buffered neutral formalin solution. They were subsequently sectioned at 5 μm and stained with hematoxylin–eosin (H&E). The sections were scored with a previously described semiquantitative scale designed to evaluate the degree of renal damage (tubular cell necrosis, cytoplasmic vacuole formation, hemorrhage, and tubular dilatation).Citation16 A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes. The scoring system used was 0, absent; 1, present; and 2, marked. Total histopathologic injury score per kidney was calculated by addition of all scores. Blind analysis of the histological samples was performed by two independent experts.
Statistical analysis
All statistical analyses were carried out using SPSS statistical software (SPSS for Windows, Version 15.0, Chicago, IL, USA). Differences in measured parameters among the three groups were analyzed by Kruskal–Wallis test. Dual comparisons between groups that present significant values were evaluated with Mann–Whitney U-test. Statistical significance was accepted a value of p < 0.05.
RESULTS
Kidney function tests
The results presented in show that treatment of rats with 1 g/kg APAP orally produced, 48 hours later, significant increase in creatinine and urea levels (p < 0.01). Treatment of rats with ozone/oxygen mixture at a dosage of 0.7 mg/kg via the intraperitoneal route for single dose significantly decreased serum creatinine and urea levels (p < 0.01).
TABLE 1. Kidney function test results of the groups (median ± SD)
Oxidative stress marker and antioxidant enzyme activities
Single-dose oral administration of 1 g/kg APAP to rats resulted in a significant increase in tissue MDA level and significant decrease in antioxidant enzyme activities (SOD and GSH-Px) in control group (p < 0.05). Treatment of the rats with ozone/oxygen mixture at a dosage of 0.7 mg/kg resulted in an significant decrease in tissue MDA levels and increase in the tissue antioxidant enzyme activities (p < 0.05) (SOD and GSH-Px) ().
TABLE 2. Oxidative stress and antioxidant enzyme levels in kidney (median ± SD)
Histopathological examination
The histopathological examination of kidneys in the sham group showed normal morphology of the renal parenchyma with well-preserved glomeruli and tubules (). In control group, total injury score was significantly higher than sham- and ozone-treated groups (p < 0.05). In control group, severe histopathological changes including tubular epithelial degeneration, vacuolization, cell desquamation, necrosis, and cellular debris in the proximal tubules were clearly observed (). In rats treated with APAP plus ozone, despite the presence of mild tubular degeneration and epithelial vacuolization in the proximal tubules, cellular desquamation was minimal and glomeruli maintained near normal morphology compared with the APAP group (, ).
FIGURE 1. Photomicrographs of kidney from rats from sham (A), kidney sections from rats treated with a single dose of 1 g/kg APAP showing significant histopathological changes (B), and kidney sections from ozone-treated group showing beneficial effects of the ozone (C) (H&E, ×400).
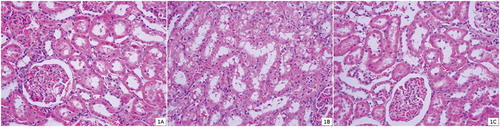
TABLE 3. Histopathologic evaluation of kidney sections for each group
DISCUSSION
This study showed that single-dose oral administration of 1 g/kg APAP to rats resulted in a significant increase in serum creatinine and urea levels. This indicates that APAP at a dose of 1 g/kg is capable of inducing severe nephrotoxicity. Ozone therapy of rats after APAP administration significantly reduced all these parameters to almost the normal range.
To our knowledge the effect of the ozone therapy on APAP-induced nephrotoxicity has not been evaluated before. In this study, it has been shown that ozone therapy has significant protective effect on single dose of APAP-induced nephrotoxicity in rats.
The mechanism of APAP toxicity is well described in the liver but is less clearly understood in the kidney. Current evidence suggests that intracellular GSH plays an essential role in detoxification of APAP and prevention of APAP-induced toxicity in the liver and kidney.Citation17,Citation18
The generation of the ROS appears as an early event which precedes intracellular GSH depletion and cell damage in APAP nephrotoxicity.Citation19 Reduced GSH is the main water-soluble cellular antioxidant that serves as the first line of defense in combating free radicals and plays a critical role in the detoxification reactions. GSH can also act as a reductant, reducing hydrogen peroxide and lipid hydroperoxides directly to H2O with the formation of oxidized glutathione (GSSG). Under conditions of continuous intracellular oxidative stress, the intracellular GSH is depleted. Depletion of intracellular GSH leads to oxidation and damage of lipids, proteins, and DNA by the ROS.Citation19,Citation20
In this study, administration of nephrotoxic doses of APAP to rats resulted in the development of oxidative stress damage in renal tissues. This effect was indicated by increasing the degree of lipid peroxidation, inhibiting the enzymatic antioxidants (e.g., GSH-Px, SOD) in kidney.
Ozone easily dissolves in biological fluids such as plasma, lymph, and urine and immediately reacts with polyunsaturated fatty acids, antioxidants, reduced GSH, and albumin. These compounds behave as electron donors and undergo oxidation, resulting in the formation of hydrogen peroxide (H2O2) and lipid oxidation products (LOPs). H2O2, an essential ROS molecule, is able to act as an ozone messenger for eliciting several biological and therapeutic effects.Citation7,Citation8,Citation10,Citation21 The dogma that H2O2 is always harmful has been changed because, in physiological amounts, it behaves as a regulator of signal transduction and is an important mediator of host defense and immune responses.Citation9,Citation21 While H2O2 acts immediately and disappears (early and short-acting messenger), LOPs, via the circulation, distribute throughout the tissues and become late and long-lasting messengers.Citation8,Citation9,Citation21 This process stimulates the innate immune system and helps the cell to survive when an injury occurs. Effects of single session of ozone therapy may last a couple of days. After cessation of ozone therapy, effects of ozone disappear. In addition, generated H2O2 during ozone therapy acts only as a second messenger and it goes to normal or previous levels immediately.Citation10,Citation21 We found that tissue SOD and GSH-Px enzyme activities were decreased in untreated rats, whereas they were increased in rats receiving ozone therapy. Furthermore, tissue MDA level (indices of tissue damage) was found to be significantly increased in the untreated group and decreased in the treatment group. Thus, ozone may be a possible beneficial effect to reduce tissue damage by enhanced antioxidant enzyme activity.
In our study, results from histopathological examination show clear evidence of nephrotoxicity after administration of an overdose of APAP. Different studies have shown that APAP-induced renal damage is consistent with acute tubular necrosis.Citation1–3,Citation22–24 Direct toxic effect of APAP on capillary wall may be responsible from APAP-induced acute tubular necrosis. The other histopathological findings of APAP-induced nephrotoxicity in rats are tubular epithelial degeneration, proximal tubular vacuolization, cell desquamation, necrosis and cellular debris in the proximal tubules, and cortical interstitial congestion.Citation1–3,Citation5 These results are also consistent with our histopathological findings. The important finding of this study was that ozone therapy ameliorated the APAP-induced severe histopathological renal changes.
Ozone therapy has ameliorative effects also on several renal dysfunctions. It was shown that ozone pretreatment prevented the increase in serum creatinine levels, the GSH depletion and the inhibition of SOD, catalase and GSH-Px activities induced by cisplatin in the rat kidney.Citation25 The protective effect of ozone is shown at the rat kidney from reperfusion injury by upregulation of antioxidant defense system and beneficial effects on blood circulation and in oxygen metabolism.Citation26
In conclusion, our study results are consistent with encouraging data for ozone therapy on APAP-induced nephrotoxicity in rats by improving antioxidant mechanism and oxidative stress. However, before its clinical use, further studies should be planned to determine the possible side effects and long-term effects of ozone therapy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Cekmen M, Ilbey YO, Ozbek E, Simsek A, Somay A, Ersoz C. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol. 2009;47(7): 1480–1484.
- Ilbey YO, Ozbek E, Cekmen M, Melatonin prevents acetaminophen-induced nephrotoxicity in rats. Int Urol Nephrol. 2009;41(3):695–702.
- Isik B, Bayrak R, Akcay A, Sogut S. Erdosteine against acetaminophen induced renal toxicity. Mol Cell Biochem. 2006;287(1–2):185–191.
- Mazer M, Perrone J. Acetaminophen-induced nephrotoxicity: Pathophysiology, clinical manifestations, and management. J Med Toxicol. 2008;4(1):2–6.
- Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM, Farrag MM. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008;243(3):261–270.
- Li C, Liu J, Saavedra JE, Keefer LK, Waalkes MP. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced nephrotoxicity in mice. Toxicology. 2003;189(3):173–180.
- Guven A, Gundogdu G, Vurucu S, Medical ozone therapy reduces oxidative stress and intestinal damage in an experimental model of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2009;44(9):1730–1735.
- Calunga JL, Zamora ZB, Borrego A, Ozone therapy on rats submitted to subtotal nephrectomy: Role of antioxidant system. Mediators Inflamm. 2005;2005(4):221–227.
- Bocci V, Borrelli E, Travagli V, Zanardi I. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med Res Rev. 2009;29(4):646–682.
- Guven A, Gundogdu G, Sadir S, The efficacy of ozone therapy in experimental caustic esophageal burn. J Pediatr Surg. 2008;43(9):1679–1684.
- Chattopadhyay RR, Sarkar SK, Ganguly S, Banerjee RN, Basu TK, Mukherjee A. Hepatoprotective activity of Azadirachta indica leaves on paracetamol induced hepatic damage in rats. Indian J Exp Biol. 1992;30(8):738–740.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358.
- Durak I, Yurtarslanl Z, Canbolat O, Akyol O. A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin Chim Acta. 1993;214(1):103–104.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169.
- Rabb H, Ramirez G, Saba SR, Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol. 1996;271(2 Pt. 2):F408–F413.
- Newton JF, Hoefle D, Gemborys MW, Mudge GH, Hook JB. Metabolism and excretion of a glutathione conjugate of acetaminophen in the isolated perfused rat kidney. J Pharmacol Exp Ther. 1986;237(2):519–524.
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10(4):267–278.
- Manov I, Hirsh M, Iancu TC. Acetaminophen hepatotoxicity and mechanisms of its protection by N-acetylcysteine: A study of Hep3B cells. Exp Toxicol Pathol. 2002;53(6):489–500.
- Abdel-Zaher AO, Abdel-Rahman MM, Hafez MM, Omran FM. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2007;234(1–2):124–134.
- Bocci VA. Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 2006;37(4):425–435.
- Boutis K, Shannon M. Nephrotoxicity after acute severe acetaminophen poisoning in adolescents. J Toxicol Clin Toxicol. 2001;39(5):441–445.
- Jones AF, Vale JA. Paracetamol poisoning and the kidney. J Clin Pharm Ther. 1993;18(1):5–8.
- Mour G, Feinfeld DA, Caraccio T, McGuigan M. Acute renal dysfunction in acetaminophen poisoning. Ren Fail. 2005;27(4):381–383.
- Borrego A, Zamora ZB, Gonzalez R, Protection by ozone preconditioning is mediated by the antioxidant system in cisplatin-induced nephrotoxicity in rats. Mediators Inflamm. 2004;13(1):13–19.
- Chen H, Xing B, Liu X, Ozone oxidative preconditioning protects the rat kidney from reperfusion injury: The role of nitric oxide. J Surg Res. 2008;149(2):287–295.
