Abstract
Chronic renal failure (CRF) is a condition associated with the risk of cardiovascular complications. Systemic inflammatory response, initiated by the pathogen-associated molecular-pattern (PAMP) molecules, exerts many similarities with the damage-associated molecular-pattern (DAMP) molecule-induced systemic response. Up to now, a number of DAMP molecules were identified. We hypothesized that the available circulating nucleic acids, acting as DAMPs, may modulate immunoinflammatory reaction in CRF. Patients with the different stages of chronic kidney disease, kidney transplantation, and patients on dialysis were included in the study. Obtained results about higher concentration of circulating ribonucleic acid (RNA), according to the stages of kidney diseases, may contribute to the hypothesis that damaged kidney tissue releases nucleic acids. Circulating RNAs expressed maximal absorbance peak at 270 nm in spectrophotometric scan analysis, which corresponded to polyC, compared to different standard samples. During in vitro conditions, by using the culture of human residential macrophages, circulating RNA isolated from patients with IV–V-stage renal diseases, patients on hemodialysis, and patients who underwent renal transplantation were able to significantly change signal transduction proteins related to inflammation and antiviral response. They significantly increased the intracellular concentration of active nuclear transcription factor nuclear factor kappa B (NF-κB), interferon regulatory factors (IRF)-3, and IRF-7 and significantly decreased melanoma differentiation-associated protein-5 (MDA-5) and p38. In this way, it seems that circulating RNA, acting as DAMP, may contribute to the mechanisms of additional inflammatory reaction, possible immune destruction, and decreased antiviral response, related to complications in kidney diseases.
INTRODUCTION
Chronic renal failure (CRF) is recognized as a common condition associated with an increased risk of cardiovascular complications, due to atherosclerotic changes.Citation1 The disease activity of many other kidney diseases, such as the IgA nephropathy, renal vasculitis, transplant rejection, lupus nephritis, and postinfectious glomerulonephritis, is linked to episodes of immunoinflammatory cascade activation.Citation2,Citation3 The systemic inflammatory response, initiated by the pathogen-associated molecular-pattern (PAMP) molecules, exerts many similarities with the damage-associated molecular-pattern (DAMP) molecule-induced systemic response.Citation4,Citation5 The “danger hypothesis” postulated that the immune system is evolved to respond to the infective agents and to “nonphysiological” cell death, damage, or stress.Citation6 Like PAMPs, DAMP-derived molecules may initiate strong inflammatory response, through the activation of antigen-presenting cells (APCs), induced production of proinflammatory cytokines [interleukin (IL)-1, tumor necrosis factor (TNF)-α, IL-6, IL-8], and the upregulated expression of cell adhesion molecules (ICAM-1, VCAM-1).Citation4–7 Up to now, a number of DAMP molecules were identified, including high mobility group box 1 (HMGB-1) protein, heat-shock proteins (HSPs), uric acid, altered matrix proteins, and S100 proteins.Citation4 These danger signals, recently known as “alarmins” and “endocrines,” mediate inflammatory responses through the toll-like receptors (TLRs), the receptor for advanced glycation end-products (RAGE), and the NOD1-like receptors, after release from activated, damaged, or necrotic cells. In response to cell damage, released DAMP molecules may activate, beside immune cells, the vascular endothelium, being in this way responsible for atherosclerotic complications, thrombotic microangiopathy, renal vasculitis, or postinfectious endocapillary proliferative glomerulonephritis.Citation8–11 Besides the role as the genetic code, nucleic acids may exert the immunomodulatory functions, when they have been recognized by a set of pattern recognition receptors. Single-stranded (ss) and double-stranded (ds) forms of nucleic acids, their synthetic analogs, or specific “danger motifs,” such as unmethylated CpG, represent strong TLR ligands and inducers. As ligands for TLR7/8 were documented the single-stranded (ss) forms, for TLR3 ds forms and for TLR9 unmethylated CpG.Citation12 It is now documented that the immunostimulatory potential of well-known adjuvants, for example, bacille Calmette–Guérin (BCG) or Freund's adjuvant, relates to nucleic acid sequence motifs in these preparations. Circulating ribonucleic acid (RNA) species, including different-sized dsRNAs and ssRNAs, may represent critical determinants for the activation of the innate immune system, whenever they are exogenic or endogenously generated.Citation7,Citation13 Introduction of dsRNA fragments into the cytoplasm may drive normal cells to become APCs.Citation14 A family of extracellular ribonucleases (RNases) is involved in degradation of all circulating RNAs or oligonucleotide derivatives.Citation12,Citation15,Citation16 Decreased activity of extracellular RNases was documented in some chronic or autoimmune diseases in our recent reports.Citation16,Citation17
We hypothesized that the available circulating nucleic acids, acting as DAMPs, may be a possible pathogenetic mechanism capable of modulating immunoinflammatory reaction in patients with CRF.
PATIENTS AND METHODS
Patients and control subjects
Patients with different stages of chronic kidney disease were recruited from the Clinic for Nephrology and Hemodialysis Medical Faculty, University of Nis. Blood laboratory markers of kidney damage, abnormalities in the composition of urine, and abnormalities in imaging tests were present in establishing a diagnosis of stages of chronic kidney disease. They were selected according to the National Kidney Foundation (NKF) criteriaCitation18: mild and moderate reduction in glomerular filtration rate (GFR) (II–III) and severe reduction in GFR and kidney failure (IV–V). Patients with kidney transplantation and patients on hemodialysis program were included in the study as well. Immunosuppressive therapy was performed in patients who underwent transplantation. It consisted of tacrolimus and prednisone. Patients were checked for history of previous or present infections as well. Patients with present acute infections were excluded. Randomly selected healthy individuals, blood donor volunteers, were enrolled in the study as the adult control group. None had above-mentioned exclusion criteria.
Isolation of nucleic acids and oligonucleotide samples
Blood samples for plasma separation were collected into heparin-containing sterile Vacutainer tubes and the blood was centrifuged for 10 min at 1800 × g according to the commercial protocol for RNA isolation. Supernatant was removed and centrifuged for a second time at 1300 × g for 10 min to eliminate any remaining cells. For the isolation of RNA from plasma samples, the commercial RNA isolation reagent set TRI Reagent BD (T3809) for the simultaneous isolation of RNA, DNA, and proteins from plasma was purchased from Sigma (St. Louis, Missouri, USA). Plasma RNA isolation was performed according to the manufacturer's instructions. Each RNA pellet was dissolved in 1000 μL of physiological saline solution. The concentration of circulating nucleic acids was calculated by using corresponding standard of RNA. Purified RNAs and related oligonucleotides were used for spectrophotometric scan analysis, explained in our previous report.Citation17 The UV spectra obtained in our study are in a close agreement with recent revised UV extinction coefficients of RNA and related nucleotides.Citation19
Preparation of Circulating Monocyte/Residential Macrophage Cell Culture
The passaged residential macrophages were obtained from the Department of Microbiology, Biochemistry, and Biotechnology, University of Maribor (Slovenia). Briefly, the peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy blood donors after Ficoll centrifugation, and they were allowed to differentiate into monocyte-derived macrophages. Dulbecco's modified Eagle medium (DMEM), supplemented with 100 μg/mL streptomycin, 100 U/mL penicillin, 10% heat-inactivated fetal calf serum, and 1 mM glutamine, was used for cultivation. For further work, residential macrophages, obtained after 64th passage, were cultured in 24-well plates, allocated into different groups: the cells exposed to media supplemented with 100 μL of physiological saline solution; the cells exposed to media supplemented with 100 μL of RNAs purified from plasma of control healthy subjects; and the cells exposed to media supplemented with 100 μL of RNAs purified from different groups of patients. The number of samples of each group corresponded to the number of circulating nucleic acid samples, except that untreated group of cells exposed to media and physiological saline solution consisted of 10 samples. The residential macrophages were cultured with isolated nucleic acids for 4 hours in incubator (Assab, Sweden) at 37°C in an atmosphere of 95% air and 5% CO2, afterwards the cells were transferred into five 96-well U-bottom plates (5 × 10Citation5 cells/well), fixed with ice-cold methanol (100%) for an additional 10 min, permeabilized with 0.1% Triton-X-100 dissolved in phosphate buffered saline (PBS), washed with PBS, and supplemented with 1% bovine serum albumin.Citation20 For determination of nuclear factor kappa B (NF-κB), p38, MDA-5, interferon regulatory factor (IRF)-3, and IRF-7, specific antibodies were purchased from Santa Cruz Biotechnology (CA, USA): for NF-κB [p65 (C-20): sc-372 epitope mapping at the C-terminus of NF-κB p65]; p38 (sc-15714); MDA-5 (C-16: sc-48031); IRF-3 (FL-425: sc-9082); and IRF-7 (sc-130509). The cells were incubated with corresponding primary antibody, washed three times, and then incubated with the fluorescein isothiocyanate (FITC)-conjugated secondary antibody. The excess of antibody, following staining, was washed by adding PBS to each well. The mean fluorescence intensity (MFI; logarithmic scale) of cell populations was determined, and the results presented here are the values of MFI following subtraction of values for unstained matched control cells, analyzed on Victor Perkin Elmer–Wallac multiplate reader. The fluorescence intensity of cells indicated for both up- and downregulating populations for each stimulus was used.
Statistical methods
The results are presented as mean ± SD. The statistical significance was evaluated between each group and control group and between groups by using analysis of variance (ANOVA) test. The limit significance for all analyses was defined as p = 0.05.
RESULTS
The concentration of circulating RNA (mg/L), isolated by using commercial RNA isolation reagent set, was significantly higher in patients in stages IV and V as well as in patients undergoing dialysis, compared to control samples (). Obtained RNA samples expressed maximal absorbance peak at 270 nm by spectrophotometric scan analysis (), which corresponded to polyC, compared to different standard samples (rRNA, DNA polyA, polyC, polyU, polyI:C, polyA:U, CpG, uric acid) (). Similar results were documented in our report, concerning the level of circulating nucleic acids in diabetic patients.Citation17
FIGURE 1. (a,b) Spectrophotometric scan analysis of plasma circulating nucleic acids and corresponding standards. For the isolation of RNA from plasma samples, the commercial RNA isolation reagent set TRI Reagent BD (T3809) was used. Purified RNAs and related oligonucleotides were employed for spectrophotometric scan analysis. For spectrophotometric scan analysis the solutions of rRNA, DNA polyA, polyC, polyU, polyI:C, polyA:U, and CpG were used. The UV spectra obtained in our study are in a close agreement with recent revised UV extinction coefficients of RNA and related nucleotides.Citation19
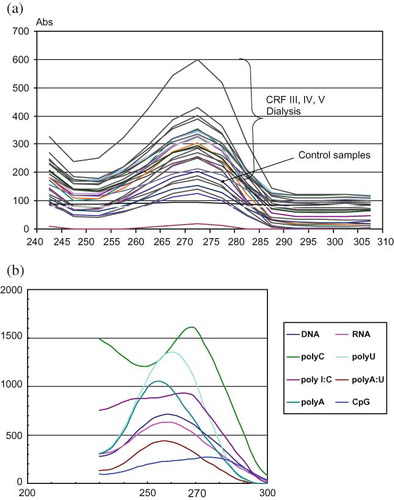
TABLE 1. Main clinical characteristics of investigated groups and circulating RNA level
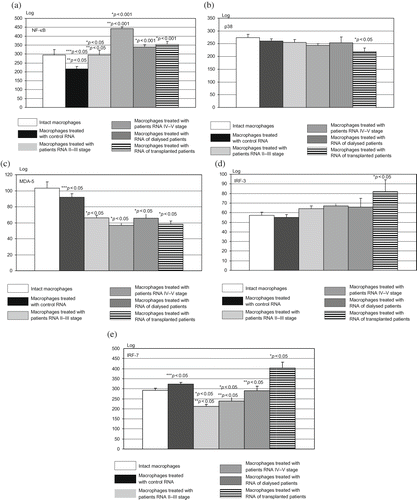
In our further research, residential macrophages were exposed to media supplemented with physiological saline or with nucleic acids isolated from control healthy subjects or patients with renal diseases. The level of NF-κB, p38, MDA-5, IRF-3, and IRF-7 was determinate in cells. Results are shown in . Residential macrophages incubated with the RNA samples from healthy subjects demonstrated significantly lower level of NF-κB and MDA-5, compared to macrophages incubated with media supplemented with physiological saline. Circulating nucleic acids isolated from patients with IV–V stage renal diseases from patients on hemodialysis and from patients who underwent renal transplantation were able to increase significantly NF-κB and to decrease significantly MDA-5 (). The level of p38 was significantly decreased only when macrophages were incubated with the RNA samples isolated from patients who underwent renal transplantation (). This group of cells demonstrated from the other side increased level of both IRF-3 and IRF-7 ().
FIGURE 2. (a–e) NF-κB, p38, MDA-5, IRF-3, and IRF-7 level after incubation of residential macrophages with isolated nucleic acids. Residential macrophages, cultured in 24-well plates, were allocated into different groups: the cells exposed to media supplemented with physiological saline solution (intact macrophages); the cells exposed to media supplemented with RNAs purified from plasma of control healthy subjects; the cells exposed to media supplemented with RNAs purified from different groups of patients. The number of samples of each group corresponded to the number of circulating nucleic acid samples, except that untreated group of cells exposed to media and physiological saline solution consisted of 10 samples. The residential macrophages were cultured with isolated nucleic acids for 4 hours, afterwards the cells were transferred into five 96-well plates for determination of NF-κB, p38, MDA-5, IRF-3, and IRF-7. The MFI (logarithmic scale) was determined, and the results presented here are the values of MFI following subtraction of values for unstained matched control cells, analyzed on Victor Perkin Elmer–Wallac multiplate reader. *p < 0.001, statistical significance compared to the cells exposed to media supplemented with RNAs purified from plasma of control healthy subjects. **p < 0.001, statistical significance compared to the cells exposed to media supplemented with RNAs purified from plasma of patients who underwent renal transplantation. ***p < 0.05, statistical significance compared to the cells exposed to media supplemented with physiological saline solution (intact macrophages).
DISCUSSION
Increased concentration of circulating nucleic acids, according to the stages of kidney disease, may contribute to the hypothesis that damaged kidney tissue releases nucleic acids. According to our hypothesis, released circulating nucleic acids may act as the DAMPs, bridging in this way kidney damage and immune cells activation. A number of examples were documented where the cell death, damage, or altered antigenic presentation has been linked to the initiation of the immunoinflammatory cascade, especially in the case of autoimmune diseases.Citation4,Citation5,Citation8,Citation10,Citation11,Citation21–23 Multiple proinflammatory events, regulated by transcription factors the NF-κB and p38, may be activated in mesangial cells and intrarenal macrophages through TLR-signaling pathways.Citation2,Citation24–28 Intrinsic renal nonimmune cells express less TLR pattern compared with intrarenal macrophages.Citation10,Citation22 The TLR 3,7–9 are capable of recognizing different-sized nucleic acids or oligonucleotides; it may allow them to sense and initiate innate and adaptive immune response in the case of nucleic acids endogenous appearance.Citation8,Citation9,Citation13 The ligand interaction of TLR7/8 activates downstream signaling cascade interleukin 1 receptor-associated kinase 4 (IRAK4) and tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), which activates NF-κB. NF-κB is the transcription factor present in the cytoplasm of every cell type in an inactive form. Upon stimulation, it is released from an inhibitory subunit (IκB) and translocates into the nucleus, promoting the transcriptional activation of many proinflammatory genes related to the inflammatory process, generating TNF-α, IL-6, IL-1β, IL-8, IL-12, IL-18, and different chemokines.Citation2,Citation3 NF-κB has also been implicated in the regulation of gene transcription that contributes to lymphocyte maturation and antigen processing and presentation by major histocompatibility complex (MHC) class I molecules. Its activation may function as a master switch in transplant tolerance.Citation29 Immunosuppressive or anti-inflammatory therapeutic strategies are aimed at modifying NF-κB activity. The p38 mitogen-activated protein kinase (MAPK) pathway is a proinflammatory signal transduction pathway responsible for the production of cytokines at cellular response to stress, bacterial lipopolysaccharide (LPS), or ischemia.Citation11,Citation20,Citation24,Citation27,Citation28 We showed that circulating RNAs may be prime resident macrophages to activate NF-κB but not p38. Activated NF-κB signaling may be responsible for the augmented inflammatory reaction and further susceptibility for vascular damage development. It was documented that the inhibition of p38, through immunosuppressive or anti-inflammatory drugs, inhibits the production of proinflammatory proteins.Citation20,Citation24,Citation27 By this mechanism, obtained decrease of p38 may be explained in patients who underwent kidney transplantation, because they are on immunosuppressive therapy. The melanoma differentiation-associated protein-5 (MDA-5) receptor together with the RIG-I-like receptor (RLR) are capable of sensing cytoplasmic dsRNAs, generated usually during viral replication. In macrophages, RLRs are the major sensors for viral infection, capable of discriminating self and nonself RNAs. The MDA-5 activates signaling through the interferon promoter stimulator 1 (IPS-1) and specific kinases TBK1 and IκB kinase epsilon (IKKϵ). The MDA-5-deficient animals exhibit a selectively impaired antiviral response.Citation30 Because MDA-5 was documented as the TLR-independent pathway, decreased MDA-5 level observed in our experiment may suggest that this signaling pathway could not be affected by endogenous nucleic acids. Decreased MDA-5 helicase activity may further contribute to impaired detection of specific viral RNAs, favoring in this way possible viral persistence and the establishment of chronic infection. Multiple TLR-dependent and TLR-independent (RIG-I and MDA-5) pathways are involved in the cell-specific regulation of type I interferons (IFNs) synthesis to ensure the activation of antiviral response.Citation10 IRF-3 and IRF-7 have been regarded as the master regulators of type I IFN gene activation. The expression of IRF-3 alone is sufficient for the induction of the IFNB gene, but the induction of all the IFNA subtypes in human cells requires the presence of IRF-7. It was also documented that the ratio between the IRF-3 and IRF-7 is a critical determinant for the induction of the individual IFNA subtypes in immune cells.Citation31–33 In our study, only the macrophages incubated with the nucleic acids isolated from patients who underwent kidney transplantation exerted increased level of both IRF-3 and IRF-7, accompanied with the NF-κB increase. Earlier report demonstrated that the peaks of the antiviral activity in serum samples occurred preponderantly in connection with clinical signs of renal graft rejections. It was proposed that serum IFN level may be a useful marker of incipient graft rejection.Citation34 IFN-α therapy was associated with acute or subacute renal failure of the kidney transplant patients.Citation35 Because mesangial cells do not generate type I IFNs, in normal conditions the IRF-3 pathway seems not to be relevant. But activated mesangial cells express high levels of TLR3 and respond to dsRNA exposure by developing glomerulonephritis and by activating apoptosis pathway. But how NF-κB and IRF pathways cross-talk may be a matter of further research.Citation10,Citation22
In conclusion, increased concentration of circulating RNAs in different stages of renal failure was documented. Circulating RNAs may act as the endogenous molecules capable of signaling the presence of danger to the surrounding immune cells and tissues. As the DAMP, they may contribute to the mechanisms of additional inflammatory reaction and/or to the inability of the host to completely rid itself of the virus. Specific antagonists for nucleic acid-dependent TLRs are not yet available, but it seems that in the progressive forms of renal failure the inhibition of the NF-κB would be of greatest importance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Parmar MS. Chronic renal disease. BMJ. 2002;325(7355):85–90.
- Rangan G, Wang Y, Harris D. NF-kappaB signalling in chronic kidney disease. Front Biosci. 2009;14:3496–3522.
- Ma FY, Liu J, Nikolic-Paterson DJ. The role of stress-activated protein kinase signaling in renal pathophysiology. Braz J Med Biol Res. 2009;42(1):29–37.
- Lotze MT, Zeh HJ, Rubartelli A, The grateful dead: Damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220(1): 60–81.
- Rubartelli A, Lotze MT. Inside, outside, upside down: Damage-associated molecular pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28(10):429–436.
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045.
- Foell D, Wittkowski H, Roth J. Mechanisms of disease: A “DAMP” view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3(7):382–390.
- Barrat FJ, Meeker T, Gregorio J, Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Environ Monit. 2005;202(8):1131–1139.
- Navab M, Gharavi N, Watson AD. Inflammation and metabolic disorders. Curr Opin Clin Nutr Metab Care. 2008; 11(4):459–464.
- Pawar RD, Patole PS, Wörnle M, Anders HJ. Microbial nucleic acids pay a Toll in kidney disease. Am J Physiol Renal Physiol. 2006;291:F509–F516.
- Mollen KP, Rahul JP, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: Toll like receptor 4- sentinel for the detection of tissue damage. Shock. 2006;26(5):430–437.
- Diebold SS, Kaisho T, Hemmi H, Akira S, Sousa R. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531.
- Deane AJ, Bolland S. Nucleic acid-sensing TLRs as modifiers of autoimmunity. J Immunol. 2006;177:6573–6578.
- Moriyama H, Wen L, Abiru N, Induction and acceleration of insulitis/diabetes in mice with a viral mimic (polyinosinic-polycytidilyc acid) and an insulin self-peptide. Proc Natl Acad Sci U S A. 2002;99:5539–5544.
- Schein CH. From housekeeper to microsurgeon: The diagnostic and therapeutic potential of ribonucleases. Nature Biotechnol. 1997;15:529–536.
- Kocic G, Bjelakovic G, Saranac LJ, Altered degradation of circulating nucleic acids and oligonucleotides in diabetic patients. Diabetes Res Clin Pract. 2008;79(2):204–213.
- Kocic G, Kocic R, Pavlovic R, Possible impact of impaired double-stranded RNA degradation and nitrosative stress on immunoinflammatory cascade in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2009;117:1–6.
- National Kidney Foundation – K/DOQI. Clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis. 2002;39(Suppl. 1):S1–S266.
- Cavaluzzi M, Borer P. Revised UV extinction coefficients for nucleoside-5′-monophosphates and unpaired DNA and RNA. Nucleic Acids Res. 2004;32:e1–e13.
- Reimer T, Brcic M, Schweizer M, Jungi TW. Poly(I:C) and LPS induce distinct IRF3 and NF-κB signaling during type-I IFN and TNF responses in human macrophages. J Leukoc Biol. 2008;83:1249–1257.
- Gelman AE, Zhang J, Chol Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+T cell survival. J Immunol. 2004;172:6065–6073.
- Wardle EN. Toll-like receptors and glomerulonephritis. Saudi J Kidney Dis Transpl. 2007;18:159–172.
- Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: All roads lead to type 1 interferons. Curr Opin Immunol. 2006;18:676–682.
- Lang KS, Recher M, Junt T, Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145.
- Konda R, Sugimura J, Sohma F, Katagiri T, Nakamura Y, Fujioka T. Over expression of hypoxia-inducible protein 2, hypoxia-inducible factor-1alpha and nuclear factor kappa B is putatively involved in acquired renal cyst formation and subsequent tumor transformation in patients with end stage renal failure. J Urol. 2008;180(2):481–485.
- Seimon TA, Wang Y, Han S, Macrophage deficiency of p38α MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119(4):886–898.
- Maung AA, Fujimi S, Miller ML, MacConmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573.
- Toshiro K, Hiroki Y, Hiroaki S, Kentaro K, Toshiko T, Noriyuki T. Role of p38 mitogen-activated protein kinase pathway on renal failure in the infant rat after burn injury. Shock. 2004;21(6):535–542.
- Tsoulfas G, Geller DA. NF-κB in transplantation: Friend or foe? Transpl Infect Dis. 2001;3(4):212–219.
- Kato H, Takeuchi O, Sato S, Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105.
- Yeow WS, Au WC, Lowther WJ, Pitha PM. Downregulation of IRF-3 levels by ribozyme modulates the profile of IFNA subtypes expressed in infected human cells. J Virol. 2001; 75(6):3021–3027.
- Peters K, Chattopadhyay S, Sen GC. IRF-3 activation by sendai virus infection is required for cellular apoptosis and avoidance of persistence. J Virol. 2008;82(7):3500–3508.
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282(21):15325–15329.
- Claesson K, Rönnblom L, Alm G, Tufveson G. Antiviral activity appearing in serum of renal transplant recipients. Its possible relation to immunological rejection. Transplantation. 1984; 38(1):32–34.
- Rostaing L, Modesto A, Baron E, Cisterne JM, Chabannier MH, Durand D. Acute renal failure in kidney transplant patients treated with interferon alpha 2b for chronic hepatitis C. Nephron. 1996;74(3):512–516.
