Abstract
Aim: Encapsulating peritoneal sclerosis (EPS) is arguably the most serious complication of chronic peritoneal dialysis (PD) therapy with extremely high mortality rates. We aimed to establish the rates of EPS and factors associated with its development in a single center. Methods: We retrospectively reviewed the records of all our PD patients from 1 January 1989 until 31 December 2008. All suspected cases were confirmed at laparotomy. Multifactorial models adjusted for potentially confounding variables such as age and sex. Results: Eleven cases of EPS were identified giving a prevalence rate of 1.98%. Median duration on PD was substantially longer in affected versus unaffected patients (42.5 months versus 13.8 months; p = 0.0002). EPS patients had experienced a mean of 3.54 previous cases of peritonitis (1 infection per year versus 0.71 per year in unaffected patients; p = 0.075). Six patients died (54.5%) due to intra-abdominal sepsis including all five who presented with small bowel obstruction. Three patients had an omentectomy and adhesiolysis performed with a successful outcome. Conclusion: Our study reinforces the link between duration on PD and EPS. While mortality was high in our cohort, emerging surgical techniques demonstrate a favorable outcome that can be achieved even in severely affected cases.
INTRODUCTION
Encapsulating peritoneal sclerosis (EPS), also referred to as sclerosing encapsulating peritonitis, represents one of the most serious complications of chronic peritoneal dialysis (PD) therapy. It is a slowly progressive inflammatory disorder affecting the peritoneum diffusely. As the process advances, there is a risk of small bowel obstruction (SBO) due to adhesions or mural fibrosis from the adjacent sclerotic peritoneum. Ultimately the peritoneum is replaced with thickened vascularized sclerotic tissue resembling an “abdominal cocoon.”Citation1 Vascularization of the peritoneum leads to ultrafiltration failure. Mortality rates are high, ranging from 24 to 56% in different series.Citation1–3 The overall prevalence within the PD population remains low and is generally reported as less than 2%.Citation1,Citation2 In a prospective multicenter study from Japan, the incidence was evaluated from a total of 1958 patients undergoing PD in 57 centers.Citation4 The authors reported an overall incidence of 2.5%. In all series, the incidence of EPS increases with duration of PD and is rare in patients treated with PD for less than 2 years.Citation3–6 There exists no data on EPS in Irish PD patients. We aimed to establish the rates of EPS in our PD population and perform a general assessment of the clinical presentation, diagnosis, management, and outcome of affected patients.
MATERIALS AND METHODS
Subjects
Our institution is the largest renal center in the Republic of Ireland. The number of prevalent PD patients treated is generally 45–60. All incident patients maintained on PD for at least 90 days from January 1989 until 31 December 2008 were included in the study. All patients used Baxter PD systems with lactate-based fluid regimes. A diagnosis of EPS was based upon either radiological or surgical evidence within the clinical context or SBO with a thickened peritoneum in the absence of an alternative etiology.
Data collection
We retrospectively collected patient demographic and clinical data from our renal patient database (Clinical Vision 3.4a Version 1.1.34.1). All demographic and clinical patient information is contained on the database. Data were sought regarding prevalence of EPS, duration of PD, frequency of peritonitis, microbiological cause of peritonitis, relevant surgical findings, and patient outcome.
Statistical analysis
Rank sum (Wilcoxon) and Fisher exact tests were used to compare demographic variables between affected and unaffected patients. Multifactorial models adjusted for the confounding variables of age and sex. Logistic regression was used to determine whether duration on dialysis and infection rates were significantly associated with EPS. Kaplan–Meier analysis was used to illustrate the estimated time to event. p-Values less than 0.05 were deemed to be statistically significant. Analysis was performed using Stata, version 8 (StataCorp, College Station, Texas, USA).
RESULTS
There were 615 patients who were treated with PD since January 1989. Eleven cases of EPS were identified giving a prevalence rate of 1.79%. Median follow-up time from commencement of PD was 67.5 months. All cases of EPS were diagnosed between 2002 and 2008. Two cases were diagnosed in patients after successful renal transplantation and another three had recently transferred to hemodialysis.
Patient characteristics
Mean age at commencement of PD was 43.3 years (±14.4 years) in those who did develop EPS versus 50.2 years (±14.1 years) in those who did not (p = 0.474). Median duration on PD was substantially longer in affected versus unaffected patients (42.5 versus 13.8 months; p = 0.0002). Five of the EPS patients (45.5%) were male compared to the rest of the PD population which was 58.6% male. The causes of end-stage renal disease (ESRD) included glomerulonephritis (n = 4), obstructive nephropathy (n = 2), and one case each of chronic pyelonephritis, interstitial nephritis, polycystic kidney disease, renal dysplasia, and unknown cause.
EPS rates
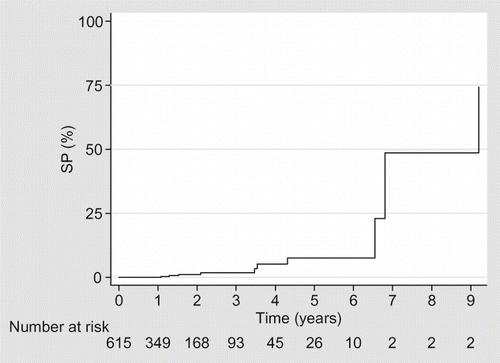
No patient developed EPS within the first year of PD treatment. However, after 6 years of PD treatment, the prevalence was 20%. Of the three patients who remained on PD for more than 8 years, all developed EPS (). The duration on PD was independently associated with developing EPS in our patient population ().
TABLE 1. Multifactorial model to determine risk of EPS
Peritonitis
There were in total 39 peritonitis infections in our 11 EPS patients (3.54 cases per patient), giving rates of 1.00 infection per patient year compared to 0.71 infections per patient year in unaffected patients (p = 0.075). The pathogens implicated were gram-positive organisms (47.8%), gram-negative organisms (26.1%), fungal (8.7%, all Candida), and culture negative (17.4%). At time of presentation with EPS, three patients had active peritonitis.
Presentation
Symptomatic SBO was the presenting event in five patients (45%). A milder presentation with gastroenterological symptoms occurred in two patients. Ultrafiltration failure was the presenting symptom in three cases. One patient was diagnosed during investigation of raised inflammatory markers. Five patients (45%) presented after switching modality of renal replacement therapy (two post-transplantation and three after commencing hemodialysis).
Diagnosis
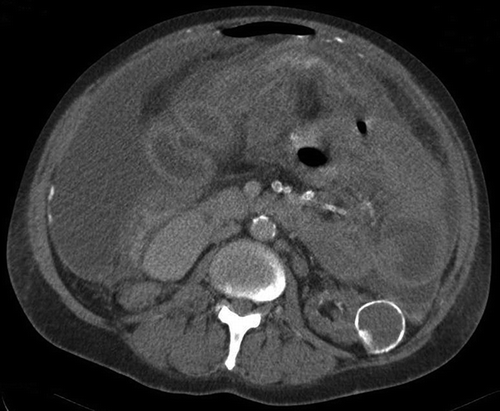
Abdominal CT scanning (performed in nine patients) supported the clinical suspicion of EPS in all cases. The CT findings showed a spectrum of changes including fluid collections thickening of the peritoneum, abscess formation, and cocooned bowel (). Two patients had abdominal ultrasound scanning and five had plain abdominal films, all of which demonstrated dilated small bowel loops. No specific radiological findings corresponded with disease severity. The diagnosis was confirmed surgically in all cases. Laparotomy findings included thickened peritoneum (in all cases), extensive encapsulated collections with cocooned bowel (n = 4), and hemoperitoneum (n = 1). Peritoneal biopsies, taken in seven cases (64%), all demonstrated the classic pathologic features of EPS.
Management
All patients changed dialysis modality as the mainstay of therapy. Nutritional support was provided in eight (73%) of our patients of which seven required total parenteral nutrition. Three patients received immunomodulatory therapy [two patients were administered prednisolone (1 mg/kg/day) and tamoxifen was used in one patient].
A range of surgical procedures were performed (55% of patients), dictated by laparotomy findings and prevalent surgical knowledge. Peritoneal lavage was undertaken in two cases and one patient had an end ileostomy created. Three patients were referred to a surgical center outside of the country where they had omentectomy and adhesion lysis performed. These three patients continue to do well and have independent gastrointestinal function. Two patients are maintained on HD and one has recently been successfully transplanted.
Outcome
Six patients died due to complications of EPS, giving a mortality rate of 54.5%. Those presenting with SBO (n = 5) had a 100% mortality rate. In four patients, the diagnosis of EPS was made directly before death. All patients died as a consequence of intra-abdominal sepsis.
DISCUSSION
Our study demonstrated an overall prevalence of EPS in our institution of 1.98%; this is consistent with rates reported in other countries.Citation1–6 As in other series, the effect of duration of PD on the development of EPS was clearly demonstrated in our cohort. No cases of EPS were identified until 2002, 13 years after the inception of our PD program. This raises the possibility that some diagnoses were missed as one would have expected that by the mid-1990s, some cases of EPS would have presented. While it is likely that some cases were not diagnosed it must be borne in mind that during this era waiting times for renal transplantation were considerably less than 12 months in Ireland. Therefore, few patients would have been maintained on long-term PD.
Over 45% of patients were diagnosed with EPS after switching to hemodialysis or receiving a transplant; this observation is consistent with the published literature.Citation7,Citation8 Some clinicians feel that the development of EPS post-transplantation may be counter-intuitive given that these patients are immunosuppressed. Indeed, there are reports that corticosteroids may have a beneficial effect on post-kidney transplantation EPS.Citation9 However, it has been theorized that cessation of peritoneal dialysis may allow fibrin, pro-inflammatory, and pro-fibrotic mediators to proliferate in the peritoneal space causing progression of sclerosing peritonitis.Citation10 Moreover, calcineurin inhibitors, the mainstay of transplant immunosuppression have pro-fibrotic characteristics.
Our study also examines the association between previous episodes of peritonitis and the subsequent development of EPS. It has been proposed that EPS may be precipitated by bacterial or fungal peritonitis on the background of an appropriately primed peritoneal membrane which has already undergone extensive epithelial mesenchymal transformation (EMT).Citation11–13 In our cohort, affected patients experienced more episodes of peritonitis than unaffected patients, although this did not reach statistical significance. Notably, there was a high rate of previous fungal peritonitis in the EPS group.
Overall, our patients presented late with a high rate of bowel obstruction and subsequent poor prognosis. A major goal in tackling the problem of EPS is to detect it at an early stage before severe peritoneal involvement has occurred. CT scanning clearly demonstrated that the classic finding of EPS in all of our patients who had it performed, although the scans took place when peritoneal sclerosis was well established. Peritoneal effluent biomarkers, including CA-125 and inflammatory cytokines, have been proposed as a potential mechanism for early diagnosis of EPS.Citation14 However, a clear relationship between any of these potential markers and EPS has yet to be demonstrated. Therefore, at present we are left with CT scanning and our clinical suspicion to attempt to identify potential early cases.
In terms of management strategies, the mainstay of treatment at our center was supportive, consisting of a change in dialysis modality and provision of parenteral nutrition in a majority of patients. The surgical techniques employed varied considerably, reflecting the evolution of modern surgical procedures. The three patients who underwent omentectomy and adhesion lysis had successful outcomes with no relapse of symptoms and no longstanding nutritional difficulties. Historically, surgical intervention was not favored due to high rates of failure.Citation15,Citation16 As demonstrated in our cohort, with modern surgical techniques patient outcome has significantly improved. Kawanishi et al. reported on 50 patients with EPS who had surgical ablation of the encapsulating membrane.Citation17 Two patients died due to surgical complications but the remaining patients all had a successful outcome.
EPS remains a life-threatening consequence of long-term PD therapy with the risk of EPS being strongly associated with the duration of PD therapy. Despite the increase in live donor transplantation rates, renal transplant waiting times continue to lengthen,Citation18,Citation19 moreover modern automated PD techniques largely circumvent the consequences of ultrafiltration failure associated with continuous ambulatory peritoneal dialysis (CAPD).Citation20 As a consequence, for the foreseeable future we can anticipate increasing dialysis vintage in the general PD population. A commensurate rise in EPS incidence can be anticipated unless preventative strategies can be effectively developed. If validated, biomarkers to identify mesothelial EMT promise early detection of “at-risk” patients and would enable a timely change in dialysis modality. Failing this, some authors suggest considering an elective switch to hemodialysis after a designated period on PD.Citation21 Clinicians must maintain a high index of suspicion to enable a prompt diagnosis of EPS. We should be particularly attentive to the long-term PD patient presenting with nonspecific gastroenterological symptoms or insidious signs of low-grade systemic inflammation. Our study further illustrates the very poor outcome of patients with this condition and highlights the importance of early detection and preventive measures. In light of its potential morbidity and mortality risk, we recommend that patients are formally counseled about EPS prior to commencing PD. Moreover, our prevalent PD population should be informed about this potentially serious complication.
There are some limitations to this study. Firstly, we acknowledge the inherent weaknesses of any retrospective, single-center study. Also, the delay in the first diagnosis from the commencement of our PD program would suggest that some cases may have been missed. However, our rates of EPS are consistent with other reports in the literature
At our center since the beginning of our program, we have demonstrated a low rate of EPS consistent with other reports. Our study reinforces the link between duration on PD and EPS. While mortality was high in our cohort, emerging surgical techniques demonstrate that a favorable outcome can be achieved even in the severely affected cases.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Dobbie JW. Pathogenesis of peritoneal fibrosing syndromes (sclerosing peritonitis) in peritoneal dialysis. Perit Dial Int. 1992;12(1):14–27.
- Kim BS, Choi HY, Ryu DR, Clinical characteristics of dialysis related sclerosing encapsulating peritonitis: Multi-center experience in Korea. Yonsei Med J. 2005;46(1):104–111.
- Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: A report of the Japanese sclerosing encapsulating peritonitis study group. Am J Kidney Dis. 1996;28(3):420–427.
- Rigby RJ, Hawley CM. Sclerosing peritonitis: The experience in Australia. Nephrol Dial Transplant. 1998;13(1):154–159.
- Kawanishi H, Kawaguchi Y, Fukui H, Encapsulating peritoneal sclerosis in Japan: A prospective, controlled, multicenter study. Am J Kidney Dis. 2004;44(4):729–737.
- Brown MC, Simpson K, Kerssens JJ, Mactier RA, Scottish Renal Registry. Encapsulating peritoneal sclerosis in the new millennium: A national cohort study. Clin J Am Soc Nephrol. 2009;4(7):1222–1229.
- Kawanishi H, Moriishi M. Epidemiology of encapsulating peritoneal sclerosis in Japan. Perit Dial Int. 2005;25(Suppl. 4): S14–S18.
- Fieren MW, Betjes MG, Korte MR, Boer WH. Posttransplant encapsulating peritoneal sclerosis: A worrying new trend? Perit Dial Int. 2007;27:619–624.
- Dejagere T, Evenepoel P, Claes K, Kuypers D, Maes B, Vanrenterghem Y. Acute-onset, steroid-sensitive, encapsulating peritoneal sclerosis in a renal transplant recipient. Am J Kidney Dis. 2005;45:e33–e37.
- Margetts PJ, Kolb M, Yu L, Inflammatory cytokines, angiogenesis, and fibrosis in the rat peritoneum. Am J Pathol. 2002;160:2285–2294.
- Lin CY, Chen WP, Yang LY, Chen A, Huang TP. Persistent transforming growth factor-beta 1 expression may predict peritoneal fibrosis in CAPD patients with frequent peritonitis occurrence. Am J Nephrol. 1998;18(6):513–519.
- Yanez-Mo M, Lara-Pezzi E, Selgas R, Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403–413.
- Selgas R, Bajo A, Jiménez-Heffernan JA, Epithelial-to-mesenchymal transition of the mesothelial cell – Its role in the response of the peritoneum to dialysis. Nephrol Dial Transplant. 2006;21(Suppl. 2):ii2–ii7.
- Schmidt DW, Flessner MF. Pathogenesis and treatment of encapsulating peritoneal sclerosis: Basic and translational research. Perit Dial Int. 2008;28(Suppl. 5):S10–S15.
- Kittur DS, Korpe SW, Raytch RE, Smith GW. Surgical aspects of sclerosing encapsulating peritonitis. Arch Surg. 1990;125:1626–1628.
- Assalia A, Schein M, Hashmonai M. Problems in the surgical management of sclerosing encapsulating peritonitis. Isr J Med Sci. 1993;29:686–688.
- Kawanishi H, Watanabe H, Moriishi M, Tsuchiya S. Successful surgical management of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25(Suppl. 4):S39–S47.
- Gaston RS, Danovitch GM, Adams PL, The report of a national conference on the wait list for kidney transplantation. Am J Transplant. 2003;3(7):775–785.
- UK renal registry report 2007 Chapter 11. Available at http://www.renalreg.com/Report-Area/Report%202007/chapter11.pdf. Accessed April 2, 2010.
- Brown EA, Davies SJ, Rutherford P, Survival of functionally anuric patients on automated peritoneal dialysis: The European APD outcome study. J Am Soc Nephrol. 2003; 14(11):2948–2957.
- Hendriks MP, de Sévaux RG, Hilbrands LB. Encapsulating peritoneal sclerosis in patients on peritoneal dialysis. Neth J Med. 2008;66(7):269–274.