Abstract
A case of immunoglobulin A nephropathy (IgAN) complicating a 10-year history of biopsy-proven Crohn's disease in a 31-year-old man is described. The patient presented with mild proteinuria and impaired renal function in the setting of an exacerbation of Crohn's disease. Renal biopsy showed IgAN. The patient responded to steroid treatment with clinical remission of the bowel disease and improvement of renal function, while proteinuria remained unchanged. IgA glomerulonephritis is rarely associated with Crohn's disease with only a few previously described cases. We briefly review these cases together with an overview of potential pathophysiological connections between these two diseases.
INTRODUCTION
The extraintestinal manifestations of inflammatory bowel disease (ulcerative colitis and Crohn's disease) are numerous and include dermatologic, arthritic, hepatobiliary, ocular, and renal or urologic complications. These manifestations typically occur in the context of clinically active intestinal disease.Citation1 The most common renal and urologic complications are related to calcium oxalate or urate stones and consequent ureteral obstruction, cystitis, enterovesical fistulas, and acute tubular necrosis, usually due to volume depletion. Renal parenchymal disease is rather uncommon although there have been reports of tubulointerstitial nephritis (TIN) and AA amyloidosis in association with inflammatory bowel disease. In particular, immune-complex glomerulonephritis is a rarely described extraintestinal manifestation of inflammatory bowel disease, with a limited number of reported cases in the worldwide literature.Citation2
Immunoglobulin A nephropathy (IgAN), recognized worldwide as the most common pattern of glomerulonephritis in individuals of all ages and particularly prevalent among those of Asian or Caucasian race, presents, in the majority of cases, as a primary disease. However, associations between IgAN and other diseases have sporadically been described, most commonly associated with liver disorders or mucosal inflammation of the gastrointestinal tract. Although a causal link is often not obvious, these cases are considered as secondary forms of IgAN.Citation3 In this report, we describe a rare case of an adult with IgAN in the setting of Crohn's disease. Additionally, we briefly review previously reported cases of glomerulonephritis associated with Crohn's disease in the worldwide literature and discuss whether these reports represent chance associations or common pathogenic pathways.
CASE REPORT
Α 31-year-old Caucasian male patient with a 10-year history of biopsy-proven Crohn's disease treated currently with methylprednisolone was referred to our nephrology outpatient clinic on 30 November 2007, with proteinuria, increased serum blood urea nitrogen (BUN), and creatinine detected at his regular laboratory examination by the gastroenterology service.
The diagnosis of Crohn's disease has been established since 1998. Colonoscopy results showed active colitis with aphthous ulcers and segmental involvement of the colon with areas of normal-appearing mucosa adjacent to inflamed mucosa, while the small intestine was unaffected. Since diagnosis and until May 2004 the disease showed twice symptomatic, biopsy-proven flare-ups that responded well to treatment with daily oral methylprednisolone, 32 mg, tapered slowly thereafter, and 5-aminosalicylic acid (5-ASA), mesalazine, 3 g/day. At regular laboratory follow-up examination in 2003, an increase in serum BUN to 27 mg/dL (9.6 mmol/L) and creatinine to 1.7 mg/dL (150 μmol/L) was noted, 5-ASA was discontinued, and the patient was referred for nephrology consultation that he missed. During the following years Crohn's disease was in clinical remission without any medical treatment, and the patient had no medical follow-up. On November 2007, he experienced a clinical exacerbation with fever, abdominal pain, and non-bloody diarrhea. Repeat colonoscopy revealed findings consistent with active Crohn's disease and the patient received a course of oral methylprednisolone. Simultaneously, proteinuria (2+ by a dipstick method) was detected in association with further deterioration of his renal function with creatinine 2.0 mg/dL (176 μmol/L) and BUN 24 mg/dL (8.59 mmol/L). The patient was urgently referred to our nephrology department for further evaluation.
Apart from inflammatory bowel disease, the patient had no significant past medical history and his only medication on nephrology consultation was oral methylprednisolone at a daily dose of 32 mg. He reported smoking about 8–10 cigarettes/day and had no history of drug or alcohol abuse. Upon admission, he was found normotensive (120/80 mmHg), afebrile, and his daily urine output ranged between 1.5 and 2.0 L. No macroscopic hematuria or upper respiratory infection episodes were reported. There was a history of upper respiratory allergies. Physical examination was unremarkable. Family history revealed a father with mild diabetes mellitus, treated by diet. Abdominal ultrasonographic and chest radiographic examinations were normal. Renal ultrasound showed normal-sized kidneys with regular contours and no signs of obstruction.
Laboratory data showed hemoglobin level at 14 g/dL (140 g/L), platelet count at 333 × 103/μL (333 × 109/L), white blood cell count at 15 × 103/μL (15 × 109/L) with 72.1% neutrophils, 18.7% lymphocytes, 6.8% monocytes, and 0.5% eosinophils. Serum creatinine (Cr) was 1.8 mg/dL (158 μmol/L) and BUN 42 mg/dL (15 mmol/L). Serum albumin and electrolyte levels were normal. Serum liver function tests, prothrombin time (PT), and partial thromboplastin time (PTT) were also normal. Urinalysis at the time showed proteinuria 2+, 3–5 red, and 2–4 white blood cells per high-power field (HPF). Urine culture showed no growth. 24-h urine measurement revealed proteinuria of 0.5 g and creatinine clearance (CrCl) of 53 mL/min/1.73 m2. Estimated glomerular filtration rate (GFR) by modification of diet in renal disease (MDRD) equationCitation4 was 60 mL/min. Serum protein electrophoresis showed increased beta and gamma globulins without peak. Urine immunoelectrophoresis confirmed the presence of nonselective proteinuria and excluded any monoclonal component.
Further laboratory tests revealed increased serum IgA of 449 mg/dL (70–410 mg/dL), IgG 1390 mg/dL (690–1600 mg/dL), IgM 125 mg/dL (34–240 mg/dL), complement component 3 (C3) levels of 129 mg/dL (90–163 mg/dL), complement component 4 (C4) levels of 31.6 mg/dL (16–44 mg/dL). C-reactive protein (CRP) was 14 mg/L. Tests for antinuclear antibody, double-stranded DNA, antimitochondrial antibodies, rheumatoid factor, antineutrophilic cytoplasmic and perinuclear antibodies, anti-Sm, anti-SSA (Ro), anti-SSB (La), and anti-RNP antibodies were all negative. Serologic tests for hepatitis B and C and HIV were also negative, thyroid function was normal, and embryonic markers (alpha-fetoprotein, carcinoembryonic antigen, CA 19-9, prostatic specific antigen) were also negative.
The working diagnosis at the time was TIN due to prior salicylate therapy or IgAN. Meanwhile, the bowel disease responded well to short steroid treatment. After steroid withdrawal at the beginning of 2008, laboratory evaluation showed stable renal function with BUN 28 mg/dL (10.02 mmol/L) and serum creatinine 1.6 mg/dL (140.8 μmol/L).
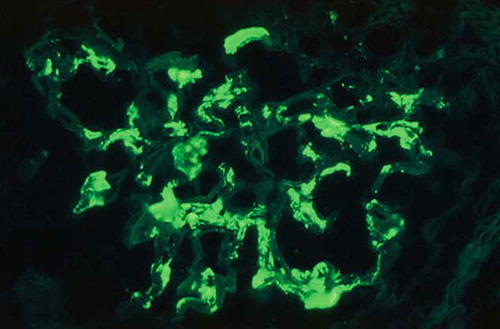
A left kidney percutaneous biopsy was performed and 28 glomeruli were found in the biopsy specimen with 12 of them (42.8%) completely fibrotic. In the remainder, light microscopy revealed moderately increased mesangial matrix and, to a lesser extent, proliferation of mesangial cells. Small- and medium-sized vessels showed moderate intimal fibrosis, while areas of interstitial fibrosis, interstitial infiltration, mainly composed of lymphocytes, tubular atrophy, and degenerative changes of tubular epithelial cells were also found (). Immunofluorescence examination included 3–5 glomeruli in every section, which showed moderate (++) to intense (+++) granular staining for IgA and C3 in the mesangium and, occasionally, in the peripheral capillary loops. Moderate (++) staining was detected for IgM and minimal staining for λ light chain. The above-mentioned findings of light microscopy and immunofluorescence examination were consistent with the diagnosis of IgAN ().
FIGURE 1. Light microscopy of a glomerulus with increased mesangial matrix and, to a lesser extent, proliferation of mesangial cells as well as areas of interstitial fibrosis and interstitial infiltration (hematoxylin–eosin stain, original magnification ×40).
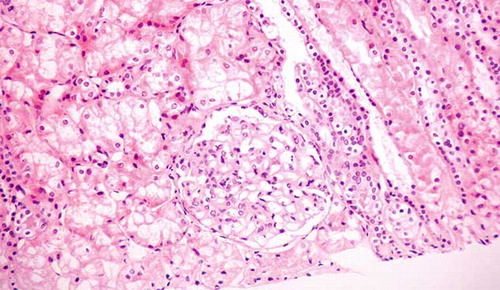
FIGURE 2. Direct immunofluorescence microscopy of a glomerulus showing intense granular staining for IgA in the mesangial areas (original magnification ×40).
Biopsy follow-up was uncomplicated and the patient was discharged in good clinical condition, normotensive (110–120/70–80 mmHg) with serum creatinine at 1.4 mg/dL (123 μmol/L), and stable proteinuria at 0.4 g/24 h. Serum IgA had decreased to 259 mg/dL. Omega-3 polyunsaturated fatty acids at a daily dose of 3 g, low protein, and salt diet were prescribed. Angiotensin-converting enzyme (ACE) inhibitors were avoided because of nonsignificant proteinuria and low blood pressure readings. On last follow-up visit, 20 months after initial presentation, the patient remained asymptomatic, with normal blood pressure, proteinuria constant at 0.5 g/24 h, stable renal function with serum creatinine at 1.3 mg/dL (114 μmol/L), and CrCl at 60 mL/min/1.73 m2.
DISCUSSION
Renal and urologic complications are reported to occur in up to 25% of patients with Crohn's disease and, like other extraintestinal manifestations, tend to be more frequent when the large intestine is involved and most often present in the setting of active bowel disease.Citation1 Most case reports, in accordance with ours, share a common feature in that the clinical course of intestinal and extraintestinal manifestations follow one another and relapse of bowel disease is commonly associated with the onset or exacerbation of extraintestinal features.Citation5 Urologic complications, such as nephrolithiasis with calcium oxalate or urate stones, intervesicular fistulas, and ureteral obstruction, appear to be more prevalent in this patient population compared to renal parenchymal manifestations.Citation2,Citation6
Intrinsic renal disease is relatively rare in association with Crohn's disease and primarily includes TIN and AA amyloidosis.Citation6,Citation7 Most reported cases of TIN associated with Crohn's disease are usually encountered in the setting of an anti-inflammatory medication use such as 5-ASA. Serious renal impairment is stated to occur in 1 out of 500 patients treated with 5-ASA derivatives.Citation8 Most cases of salicylate-induced TIN are reported within the first 12 months of exposure to the medication, but delayed presentation after several years has been shown as well.Citation8,Citation9 Additionally, symptoms and signs in most of the patients with renal disease associated with 5-ASA treatment are nonspecific making early diagnosis even more difficult. Salicylates should be withdrawn as soon as renal impairment manifests in a patient with inflammatory bowel disease. Early discontinuation of such derivatives results in the resolution of renal impairment in the majority of cases. If this does not happen, then renal biopsy should be considered. A trial of high-dose steroids may be recommended in patients whose renal function does not respond to drug withdrawal.Citation8,Citation9 Therefore, the inclusion of TIN in the differential diagnosis was justified in the present case. Consequently, 5-ASA was correctly discontinued upon renal function deterioration in 2003. Unfortunately, the patient was lost to follow-up and histological diagnosis of his underlying nephropathy or, at least, renal function evolution is missing.
Immune-complex glomerulonephritis is rather rarely described in Crohn's disease.Citation1 Eighteen cases are reported in the worldwide literature, many of which in non-English-language publications. Furthermore, a significant proportion of these cases are encountered in pediatric populations. Various histological forms of glomerulonephritis associated with Crohn's disease are described, including four cases of membranoproliferative glomerulonephritis,Citation10–13 one case of mesangial proliferative glomerulonephritis,Citation14 two cases of membranous nephropathy,Citation15,Citation16 one case of thin basement membrane disease,Citation17 one case of IgA antiglomerular basement membrane disease,Citation6 and nine cases of IgAN.Citation17–25 Renal manifestations occurred in the setting of active bowel disease in every case. Furthermore, it is of particular interest that proteinuria and renal function often improved in parallel under the treatment of the gastrointestinal disorder, suggesting a glomerulonephritis secondary to the inflammatory bowel disease, though a direct effect of the steroid treatment per se cannot be excluded.
Five out of nine IgAN cases associated with Crohn's disease are in non-English-language literatureCitation18–20,Citation23,Citation25 and three of them are pediatric cases.Citation17,Citation22,Citation23 The first report was published in 1984 by Hubert et al. and involved two patients: one with ulcerative colitis and the other with Crohn's disease, who developed glomerulonephritis due to IgA mesangial deposits.Citation18 The age distribution in the reported cases is similar to that of patients with Crohn's disease without glomerulonephritis with a median of 22 years (range 10–36 years). Hematuria was reported initially in all but one patient, with two of them exhibiting macroscopic hematuria. Proteinuria on urinalysis was also present in all but two patients. Quantitative estimation of proteinuria in timed urine samples was reported in five cases; all with values in the non-nephrotic range and 2/5 with proteinuria less than 1 g/24 hr. Renal impairment at the time of initial presentation was reported in four out of nine cases. Crohn's disease treatment included steroids and occasionally surgery, resulting in improvement of renal function in all cases. However, none regained normal renal function. Levels of proteinuria following treatment were reported in six of seven patients with proteinuria at the time of diagnosis, showing an improvement under treatment of the gastrointestinal disorder. Repeat renal biopsy, after steroid treatment, was performed in only one of the reported cases showing remission of the IgAN.
Our case represents the tenth reported case of biopsy-proven IgAN associated with Crohn's disease. In our patient, microscopic hematuria, mild proteinuria, and renal impairment were all present at the time of diagnosis of IgAN. Serum IgA concentration was also increased at this time. Steroid treatment of the bowel disease resulted in complete remission of Crohn's disease and an improvement in renal function, decrement in serum IgA concentration, and no significant effect on proteinuria or hematuria. Although steroid treatment was not associated with histological healing of IgAN in our patient, it may have had an effect on renal biopsy findings, in particular by resolving any active lesions. In our case, tubulointerstitial involvement, namely tubulointerstitial fibrosis and atrophy, were present in renal histological examination. Therefore, coexistence of IgAN with TIN due to prior 5-ASA exposure cannot be excluded, although the finding of 12 out of 28 glomeruli to be completely fibrotic is rather more consistent with the diagnosis of glomerulonephritis as primary lesion.
IgAN is considered to be a primary glomerulopathy. However, there is increasing literature reporting associations between IgAN and other diseases, in particular of the liver and the bowel. Whether these reports represent chance associations or pathophysiologically related conditions remains a matter of debate. We always have to take into consideration the relatively high frequency of subclinical IgAN in supposedly healthy populations and the issue of a chance association between IgAN and other frequent, pathophysiologically unrelated conditions.Citation3
A number of dietary and microbial antigens have been identified in circulating IgA immune complexes and mesangial IgA deposits, suggesting that environmental factors may be implicated in the pathogenesis of IgAN. Evidence from studies in familial and secondary forms of IgAN has suggested that several mechanisms can produce this pattern of glomerular morphology. However, studies searching a causal link between IgAN and specific bacterial, viral, or dietary antigen have failed to identify any single antigen responsible for the development of IgAN, although in some instances specific pathogens and food antigens have been found to drive the disease in individual patients. A representative example is the IgA antigliadin antibodies formed after oral immunization with food antigens including gliadin in patients with celiac disease, accompanied by mesangial deposition of gliadin–antigliadin immune complexes. Many of the associations reported in the literature describe environmental and microbial factors that generate pathogenic IgA in the context of primary IgAN. A variety of diseases, in particular inflammatory bowel disease and celiac disease, may impair mucocutaneous antigen exclusion and increase the contributory effect of environmental and microbial antigens.Citation3,Citation26
At present, there is no evidence supporting a pathophysiologic link between immune mechanisms operating in inflammatory bowel disease and immune mechanisms operating in IgAN. However, during exacerbations of bowel disease, a potential link between these conditions can be speculated through the possible association of a bowel disease relapse, at least in part, with the loss of mucosal antigen exclusion, due to impaired integrity of mucosal barriers, resulting in increased systemic antigenemia and subsequent increased immune activation. Moreover, the clinical course of both diseases follows one another and treatment of the bowel disease with either immunosuppression or bowel resection re-establishes mucosal antigen exclusion and is subsequently associated with a clinical remission of IgAN. Someone could argue that these observations are in favor of a common pathogenetic mechanism involving an IgA immune response to a mucosal challenge in the intestine. However, the possibility of primary IgAN that deteriorates by coexistent inflammatory bowel disease or becomes clinically evident during a relapse of the bowel disease cannot be excluded and emerges as a plausible alternative to the speculations of a common pathogenetic mechanisms between these conditions.Citation3,Citation26,Citation27
In conclusion, it is important to recognize that immune-complex glomerulonephritis, particularly IgAN, is a potential extraintestinal manifestation of Crohn's disease that occurs in the setting of active bowel inflammation. Renal impairment at initial presentation appears to be a relatively common complication in these patients. Salicylate-induced TIN should always be considered in the differential diagnosis as 5-ASA derivatives are widely used for long-term maintenance therapy in this patient population and renal histological examination is necessary under these circumstances. Physician awareness of these important interrelationships will lead to earlier detection of renal impairment and enable appropriate therapeutic measures. However, in most cases, therapy of the intestinal disease is associated with improvement in renal manifestations, although there is no clear evidence of concomitant histological remission. The pathogenesis of this association between inflammatory bowel disease and immune-complex glomerulonephritis has not been clearly elucidated and deserves further investigation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Rothfuss KS, Strange EF, Herrlinger KR. Extraintestinal manifestations and complications of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819–4831.
- Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93:504–514
- Pouria S, Barratt J. Secondary IgA nephropathy. Semin Nephrol. 2008;28:27–37.
- Tidman M, Sjöström P, Jones I. A Comparison of GFR estimating formulae based s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant. 2008;23:154–160.
- Kane S. Urogenital complications of Crohn’s disease. Am J Gastroenterol. 2006;101(12 Suppl.):S640–S643 ( Review).
- Shaer AJ, Stewart LR, Cheek DE, Hurray D, Self SE. IgA antiglomerular basement membrane nephritis associated with Crohn’s disease: A case report and review of glomerulonephritis in inflammatory bowel disease. Am J Kidney Dis. 2003;41: 1097–1109.
- Elloumi H, Ben Slama A, Arfaoui D, Ghouma M, Sfar S, Ajmi S. Renal amyloidosis complicating Crohn’s disease: Report of two cases and review of the literature. Tunis Med. 2006;84: 253–255 ( Article in French).
- Waters AM, Zachos M, Herzenberg AM, Harvey E, Rosenblum ND. Tubulointerstitial nephritis as an extraintestinal manifestation of Crohn’s disease. Nat Clin Pract Nephrol. 2008; 4:693–697.
- Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: A systematic review. Inflamm Bowel Dis. 2007;13:629–638.
- Dyduch J, Kucharska K, Depowski M, Sancewicz-Pach K. Crohn’s disease with chronic glomerulonephritis in a 14-year-old boy. Polski Tygodnic Lekarski. 1976;31:2025–2026 ( Article in Polish).
- Glassman M, Kaplan M, Spivak W. Immune-complex glomerulonephritis in Crohn’s disease. J Pediatr Gastroenterol Nutr. 1986;5:966–969.
- Presti ME, Neuschwander-Tetri BA, Vogler CA, Janney CG, Roche JK. Sclerosing cholangitis, inflammatory bowel disease and glomerulonephritis. Digest Dis Sci. 1997;42:813–816.
- Molina-Perez M, Gonzalez-Reimers E, Santolaria-Fernandez F, Maceira-Cruz B, Pavina-Cabrera M. Rapidly progressive glomerulonephritis and inflammatory bowel disease. Dis Colon Rectum. 1995;38:1006–1007.
- Schofield PM, Williams PS. Proliferative glomerulonephritis associated with Crohn’s disease. Br Med J. 1984;289:1039.
- O’Loughlin EV, Robson L, Scott B, Alexander F, Gall DG. Membranous nephropathy in a patient with Crohn’s disease of the small bowel. J Pediatr Gastroenterol Nutr. 1985;14:135–139.
- Diaz Rodriquez C, Grania E, Vazquez Martuk E, Association between membranous nephropathy and Crohn’s disease. Nephrologia. 2004;24:368–371 ( Article in Spanish).
- McCallum D, Smith L, Harley F, Yiu V. IgA nephropathy and thin basement membrane disease in association with Crohn disease. Pediatr Nephrol. 1997;11:637–640.
- Hubert D, Beaufils M, Meyrier A. Immunoglobulin A glomerular nephropathy associated with inflammatory colitis. Apropos of 2 cases. Presse Med. 1984;13:1083–1085 ( Article in French).
- Lee JM, Lee KM, Kim HW, Crohn’s disease in association with IgA nephropathy. Korean J Gastroenterol. 2008;52: 115–119 ( Article in Korean).
- Youm JY, Lee OY, Park MH, Crohn’s disease associated with IgA nephropathy. Korean J Gastroenterol. 2006;47:324–328 ( Article in Korean).
- Forshaw MJ, Guirguis O, Hennigan TW. IgA nephropathy in association with Crohn’s disease. Int J Colorectal Dis. 2005;20:463–465.
- Takemura T, Okada M, Yagi K, Kuwajima H, Yanagida H. An adolescent with IgA nephropathy and Crohn disease: Pathogenetic implications. Pediatr Nephrol. 2002;17:863–866
- Dabadie A, Gié S, Taque S, Babut JM, Roussey M. Glomerular nephropathy with IgA mesangium deposits and Crohn disease. Arch Pediatr. 1996;3:884–887 ( Article in French).
- Hirsch DJ, Jindal KK, Trillo A, Cohen AD. Acute renal failure in Crohn’s disease due to IgA nephropathy. Am J Kidney Dis. 1992;20:189–190.
- López Barbarín JM, Lafuente Martínez P, García Campos F, Ibarra Peña B, Díaz de Otazu R. Crohn’s disease associated with Berger’s disease. A rare complication. Rev Esp Enferm Dig. 1990;78:233–235 ( Article in Spanish).
- Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–2097.
- Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004;24:197–217
