Abstract
Background: Hypertension is frequently seen in autosomal dominant polycystic kidney disease (ADPKD), and it has a negative effect on renal progression. Hypertension and left ventricle hypertrophy (LVH) are related in terms of pathogenesis and their effects on renal progression. In this study, we aimed to compare the effects of losartan and ramipril on blood pressure (BP) control, LVH, and renal progression in patients with hypertensive ADPKD. Methods: Thirty-two ADPKD patients with ages ranging between 18 and 70 years who were stage 1–2 hypertensive were included in this study. Routine biochemical tests and echocardiography were obtained at first examination of the patients. Following these, the patients were randomized. One group was given losartan and the other ramipril. They were followed up for 1 year, and their echocardiographies and routine biochemical tests were repeated at the end of the year. Results: BP values decreased in both the groups at the end of the first year (p < 0.001). There was a statistically significant difference in LVH in both the groups at the end of the first year than at the beginning (losartan, p = 0.007; ramipril, p < 0.001). Conclusions: In this study, effective BP control was obtained with losartan and ramipril and LVH was found to be regressed significantly in the hypertensive patients with ADPKD. These two groups of antihypertensive drugs may also have beneficial effects on the retardation of renal progression and in reducing cardiovascular mortality in hypertensive patients with ADPKD.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is a common form of polycystic kidney disease with an incidence of 1/800. It is one of the common causes of end-stage renal disease.Citation1 Hypertension is also frequent among these patients. It is observed in 60% of these patients before the impairment of kidney functions.Citation1,Citation2 Left ventricle hypertrophy (LVH) is seen in 48% of the hypertensive polycystic kidney patients. Hypertension and LVH accelerate kidney function deterioration and cause cardiovascular morbidity and mortality.Citation3
Early diagnosis and suitable treatment of hypertension is the best approach to slow down the progression of the renal disease and reduce cardiovascular mortality. There is no consensus on the most suitable antihypertensive treatment of the hypertensive polycystic kidney patients. Although the exact mechanism of hypertension in ADPKD is not clarified yet, the most emphasized mechanism is activation of the renin–angiotensin system (RAS) because of cyst expansion and pressure on the nearby tissues as a result of ischemia.Citation4
In this study, we aimed to find out whether ramipril or losartan, which blocks the RAS in different stages, would provide effective blood pressure (BP) control in this patient group and to compare their effects on LVH.
MATERIALS AND METHODS
In this study, 32 patients with ages ranging between 18 and 70 years and with an ADPKD diagnosis according to the ADPKD diagnosis criteria,Citation5 who were stage 1–2 hypertensive according to the JNC VII, and had taken antihypertensive treatment before following the hypertension diagnosis and with creatinine clearance (Cr Cl) above 30 mL/min/1.73 m2 were included. Patients who had other renal illness or comorbidity, including diabetes mellitus (DM), congestive heart failure, liver function failure, pregnancy, lactation, or using anti-arrhythmic, oral contraceptives, immunosuppressive medicine, and steroids, and who had psychiatric disorders (those with schizoaffective conditions who could not use the drugs in the study) were not included in this study.
The study commenced after approval by the institutional ethical committee. Patients were included in the study after getting their informed consent.
Study procedure
Thirty-two patients conforming to the study criteria were accepted to the study. The patients underwent detailed physical examination. After allowing the patients to rest in the sitting position for 5 minutes, their BP values were measured twice on both arms using mercury sphygmomanometers. Patients with a BP measurement of 140/90 mmHg and above or those on hypertensive medication were included in the study. Although the antihypertensive drugs were stopped in stage 1 hypertensive patients 2 weeks before the study, the antihypertensive medication were continued in the stage 2 hypertensive patients. Echocardiographic analysis of the patients was performed with HP SONOS 5500 device using a 3.5-MHz probe and double-dimension M-Mod pictures. Left ventricle mass (LVM) was measured using the Devereux formula.Citation6
where LVEDD is left ventricle end-diastolic diameter (cm), IVS the interventricular septum (cm), PW the posterior wall (cm), Left ventricle mass index (LVMI) = LVM/body surface area (BSA), and BSA = (W0.425 × H0.75) × 0.007184 (Dubous formula).
Routine biochemical parameters were studied in the Roche Moduler auto-analyzer by using their own solutions. Cr Cl was measured by the 24 hour urine collection. Afterwards, the patients were randomized by drawing. One group was given 50 mg losartan and the other group 2.5 mg of ramipril. Patients who did not have any adverse effects and reached the required BP values according to our office BP measurements and their BP values at home every 15 days were given higher doses. Losartan (100 mg) and ramipril (5 mg first and 10 mg later) were given. The patients who did not reach the required BP values were given amlodipine 5–10 mg, doxazosin mesylate 2–4–8–16 mg, and rilmenidine dihydrogen phospate treatments, at their examinations every 2 weeks. The patients were invited to the hospital every 2 months for examination after they reached the required BP levels. At the end of 1 year, echocardiographic analysis was repeated as well as routine biochemical analyses and Cr Cl tests.
The appropriateness of the measured values was analyzed according to the Kolmogrov–Smirnov test. Variance analyses were used in the analysis of the data suitable to normal frequency. Student's t-test was used in the comparison of the two groups. The measured values were shown as arithmetic mean ± standard deviation. Statistical significance was p < 0.05.
RESULTS
The arithmetic mean of all 32 patients' demographic data, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) values, LVMI, hematocrit, creatinine, and Cr Cl were shown in .
TABLE 1. The baseline and first year values of losartan and ramipril groups
At the end of the first year in the losartan group, SBP, DBP, and MAP values were 116.58 ± 8.50, 72.89 ± 4.50, 87.41 ± 5.66 mmHg, respectively, and in the ramipril group were 120.70 ± 9.12, 74.62 ± 5.18, 89.69 ± 6.29 mmHg, respectively (p < 0.001). Both the groups reached the required BP values at the end of the first year. There were no statistically significant differences among SBP, DBP, and MAP values of the losartan and ramipril groups at the beginning and the end of the first year ().
Although the Cr Cl of the losartan group was 73.85 mL/min/1.73 m2 at the end of the first year, it was 77.25 mL/min/1.73 m2 in the ramipril group. There was no statistically significant difference between the Cr Cl of either group at the beginning and at the end of the first year. There were no significant changes in Cr Cl at the end of the first year in either group ().
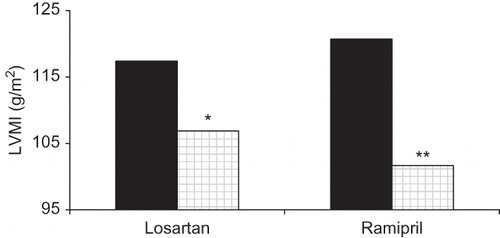
Although LVMI decreased to 106.88 ± 20.12 g/m2 in the losartan group at the end of the first year, it was 101.69 ± 15.25 g/m2 in the ramipril group. There was no statistically significant difference in the comparison of LVMI in either group at the beginning and at the end of the first year ().
DISCUSSION
Hypertension is seen in 60% of the polycystic kidney disease patients before the impairment in kidney functions.Citation1,Citation2 Hypertension is one of the most important treatable risk factors, which may have an effect on the progression of kidney functions, cardiovascular mortality, and morbidity. For this reason, importance of controlling BP is crucial.Citation3
Two groups of antihypertensive medicine blocking the RAS are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). In the Ramipril Efficacy on Nephropathy Study, Ruggenenti et al.Citation7 showed the positive effects of ramipril on BP control and renal progression in nondiabetic renal disease. In the study of Heart Outcomes Prevention Evaluation, the cardioprotective effect of ramipril was shown.Citation8 In the study of Losartan Intervention of Endpoint Reduction in Hypertension, the positive effects of losartan on cardiovascular system were indicated.Citation9
There is no agreement on the first-line antihypertensive drug choice in polycystic kidney disease. Considering the fact that the researchers claim that hypertension in polycystic kidney disease is renin dependent, the drugs that block the RAS may be more effective on the control of BP in these patients.Citation3,Citation4,Citation10–12
In our study, we gave ramipril as an initial treatment to 13 of 32 hypertensive polycystic kidney disease patients and losartan to 19 patients. Four patients in the ramipril group and seven in the losartan group needed combination therapy.
There was no significant difference between initial Cr Cl of the two groups. Although Cr Cl decreased by about 2.06 mL/min in the losartan group at the end of 1 year, the decrease was 2.8 mL/min in the ramipril group. The initial mean Cr Cl was 75.9 mL/min in the losartan group and 80 mL/min in the ramipril group. There was no significant difference in Cr Cl of the two groups at the end of the first year. In the study by Ecder et al.Citation10 on hypertensive polycystic kidney patients, enalapril and amlodipine were compared. The mean Cr Cl of the patients was about 77 mL/min in the enalapril group and 83 mL/min in the amlodipine group. The patients were followed up for 5 years, and their Cr Cl decrease was about 3.6 mL/min per year. In the study by Nutahara et al.Citation4 in Japan, the efficacy of amlodipine and candesartan on 49 hypertensive polycystic kidney patients was compared. The Cr Cl decrease in the amlodipine group was about 5.5 mL/min per year and 1.6 mL/min in the candesartan group. The mean Cr Cl of patients was 70 mL/min. Cr Cl decrease was observed to be higher when compared to the Modification of Diet in Renal Disease (MDRD) Study. However, the reason for the greater decrease might be that the Cr Cl of the patients in the MDRD study was below 55 mL/min and the follow-up period was 2.2 years.Citation13 Compared with these two studies, Cr Cl decrease was low in both of our patient groups. As the follow-up period of our study was 1 year, it was short to have an idea on Cr Cl decrease and the results of a longer follow-up study would provide a better understanding in the study.
In other studies, it was shown that LVH was higher compared to the control group before hypertension and renal damage started in polycystic kidney patients.Citation14,Citation15 In a study, compared to control group, 23% higher LVH was identified in normotensive polycystic kidney patients.Citation16 One of the mechanisms highlighted that ischemia occurs as a result of cyst pressure to nearby tissues because of expansion. The RAS activation starts at early stages of the disease before hypertension and renal damage are seen. The RAS activation plays an important role in atherosclerosis, LVH heart failure, and end stage renal failure.Citation14,Citation16 Hereditary factors and myocardial collagen matrix failure as well as RAS activation are the factors blamed for LVH etiology in normotensive polycystic kidney patients.Citation14,Citation17 In the study by Valero et al.Citation14 the contribution of ambulatory BP change to LVH in normotensive polycystic kidney patients was evaluated. It was found that there was no difference between polycystic kidney patients and control group in terms of ambulatory BP, whereas LVH was found to be higher in polycystic kidney patients. In that study, it was pointed out that the most blamed hemodynamic factor was hypertension in LVH etiology among hypertensive population, and it could not explain the increase in mass index by itself. It was indicated that nonhemodynamic factors were more effective on LVH in normotensive individuals.Citation14
In the study by Ecder et al.,Citation3 14 patients with polycystic kidney were given enalapril treatment and followed up for 7 years. It was pointed out in the study that enalapril treatment was more effective on the regression of LVH. Schrier et al.Citation18 compared the efficacy of enalapril and amlodipine treatments on the regression of LVH in hypertensive polycystic patients. In that study, it was suggested that enalapril compared with amlodipine was more effective in preventing hypertrophy, and also hypertrophy was prevented better in the group in which BP was controlled with enalapril. There is no related study on the effects of angiotensin receptor blockers on LVH in polycystic kidney patients.
In our study, at the end of the first year, LVMI was found to be significantly regressed in both the groups. The reason of regression in LVH is thought to be that these two groups of medicine-blocking RAS provided effective BP control and the patients were at the required BP values at the end of the first year. In addition, as the researchers pointed before, the RAS activation is seen during early stages of the disease in polycystic kidney patients and it has been effective on both hypertension and LVH. The drugs blocking RAS provide beneficial effects as well as BP control. BP control and RAS inhibition may have caused the regression of LVH. In our study we have found that losartan, an ARB, is a drug whose effect on LVH was not previously studied in ADPKD patients.
In conclusion, effective BP control was provided by ramipril and losartan in patients with polycystic kidney disease. To find the ideal antihypertensive drugs in polycystic kidney disease, further studies using also a combination of these two groups of drugs with a longer study period is needed.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164.
- Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342.
- Ecder T, Edelstein CL, Chapman AB, Reversal of left ventricular hypertrophy with angiotensin converting enzyme inhibition in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1999;14:1113–1116.
- Nutahara K, Higashihara E, Horie S, Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2005;99:18–23.
- Wołyniec W, Jankowska MM, Król E, Czarniak P, Rutkowski B. Current diagnostic evaluation of autosomal dominant polycystic kidney disease. Pol Arch Med Wewn. 2008;118:767–773.
- Devereux RB, Alonso DR, Lutas EM, Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–458.
- Ruggenenti P, Perna A, Mosconi L, Randomized placebo-controlled trial of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 1997;349:1857–1863.
- Yusuf S. Clinical, public health, and research implications of the Heart Outcomes Prevention Evaluation (HOPE) Study. Eur Heart J. 2001;22:103–104.
- Dahlof B, Devereux RB, Kjeldsen SE, Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomized trial against atenolol. Lancet 2002;359:995–1003.
- Ecder T, Chapman AB, Brosnahan GM, Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:427–432.
- Schrier RW, McFann KK, Johnson AM. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int. 2003;63:678–685.
- Watson ML, Macnicol AM, Allan PL, Wright AF. Effects of angiotensin converting enzyme inhibition in adult polycystic kidney disease. Kidney Int. 1992;41:206–210.
- Levey AS, Greene T, Beck GJ, Dietary protein restriction and the progression of chronic renal disease: What have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J Am Soc Nephrol. 1999;10:2426–2439.
- Valero FA, Martinez-Vea A, Bardaji A, Ambulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1999;10:1020–1026.
- Martinez-Vea A, Bardaj A, Gutierrez C, Exercise blood pressure, cardiac structure, and diastolic function in young normotensive patients with polycystic kidney disease: A prehypertensive state. Am J Kidney Dis. 2004;44:216–223.
- Oflaz H, Alisir S, Buyukaydin B, Biventricular diastolic dysfunction in patients with autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:2244–2249.
- Ecder T, McFann KK, Raynolds MV, Schrier RW. No effect of angiotensin-converting enzyme gene polymorphism on disease progression and left ventricular hypertrophy in autosomal dominant polycystic kidney disease. Am J Nephrol. 2003;23:466–470.
- Schrier R, McFann K, Johnson A, Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: Results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002;13:1733–1739.