Abstract
Background: Asymmetric dimethylarginine (ADMA) as a uremia toxin is accumulated in end-stage renal disease (ESRD) patients. Elevated ADMA level has been shown to be predictive of cardiovascular diseases (CVDs) and all-cause mortality in ESRD. Therefore, we investigated the removal of ADMA by different dialysis treatments. Methods: There were 30 each of hemodialysis (HD), hemodiafiltration (HDF), peritoneal dialysis (PD) patients, and healthy volunteers enrolled. The ADMA concentrations in serum, urine, and spent dialysate samples were determined. The urine and spent dialysate volumes were recorded. The ADMA removals by urine and spent dialysate in 1 week were calculated and compared among four groups. It was also analyzed for the correlations between the total removal of ADMA in 1 week and the parameters of age, durance of dialysis, glomerular filtration rate, urine volume, urinal ADMA level, spent dialysate volume, and spent dialysate ADMA level. Results: The serum levels of ADMA in dialysis patients were much higher than in healthy subjects (0.32 ± 0.09 μmol/L), and their 1-week total removals of ADMA were much lower than healthy controls (249.21 ± 57.04 μmol/week) (p-values all were less than 0.01). Among dialysis groups, serum ADMA levels decreased significantly in PD patients compared with HD or HDF patients (1.38 ± 0.30 μmol/L vs. 1.82 ± 0.38 μmol/L and 1.63 ± 0.32 μmol/L, p < 0.01), and the total removal of ADMA diminished remarkably by turns of PD, HDF, and HD groups (47.79 ± 8.20 μmol/week, 31.79 ± 8.92 μmol/week, 14.63 ± 6.53 μmol/week, respectively, p < 0.01). The total removal of ADMA in 1 week was related directly with the spent dialysate concentrations of ADMA, the spent dialysate volume, and the urine volume. Conclusions: ADMA was mainly removed by dialysate in dialysis patients. Different dialysis models have different clearance capability on plasma ADMA. PD might be more effective on ADMA removal than HD and HDF, with HDF being more effective than HD.
INTRODUCTION
Asymmetric dimethylarginine (ADMA) as a uremia toxin is a natural endogenous competitive NOS inhibitor. The plasma level of ADMA is increased in patients suffering from chronic kidney diseases (CKDs), because its excretion and degradation are decreased.Citation1,Citation2 The effect of ADMA on cardiovascular system, along with endothelial dysfunction and endothelial injury, is mainly through NOS–NO pathway, that is, a reduction in the production or effect of NO caused by accumulation of ADMA.Citation3—5 More recently, ADMA levels have been shown to be predictive of cardiovascular diseases (CVD) and all-cause mortality in predominantly nondiabetic CKD,Citation6 type 1 diabetic patients with diabetic nephropathy,Citation7 end-stage renal disease (ESRD),Citation8 and coronary artery disease.Citation9—12 Therefore, it is very important for ESRD patients to remove ADMA effectively.
Therefore, we investigated the circulating ADMA levels, glomerular filtration rate (GFR), and the removal of ADMA by different dialysis treatments and by urine in dialysis patients.
MATERIALS AND METHODS
Patients
Patients were selected randomly from a pool of 446 dialysis patients in one blood purification center, which included one hemodialysis (HD) union (including 250 patients) and one peritoneal dialysis (PD) center (including 196 patients). There were 30 maintenance HD patients, 30 hemodiafiltration (HDF) patients, 30 continuous ambulatory peritoneal dialysis (CAPD) patients, and 30 healthy volunteers involved in this study. The demographic and clinical characteristics of dialysis patients and controls are listed in . The drug therapy was identical in dialysis patients, included recombinant human erythropoietin (rHuEPO), hypotensive drugs, calcitriol, ferrous succinate tablets, etc. The prescriptions of drugs that might affect the metabolism of ADMA, such as vitamin B, vitamin E, folic acid, arginine, and statins, were not be found in the patients during this study. These patients had never transferred to other dialysis methods since they began renal replacement treatment. Patients with pulmonary diseases, congestive heart dysfunction, tumor, ongoing infection, or inflammatory diseases were excluded from the study. The study was approved by the Ethics Committee of the Beijing Friendship Hospital affiliated to Capital Medical University.
TABLE 1. Demographic and clinical characteristics of dialysis patients and controls
Methods of dialysis and sample collection
All the HD patients were dialyzed with polysulfone dialyzer (1.3 m2, ultrafiltration coefficient = 5.5 mL/h mmHg, Hemoflow, F6; Fresenius Medical Care AG, Bad Homburg, Germany) 3 times per week and 4 hours every session. Blood and dialysate flow rates were 285 ± 34 mL/min and 496 ± 11 mL/min, respectively. The urea clearance index (KT/V) was larger than 1.2 per session. Dialysate composition was as follows: sodium (Na+) 135 mM/L, bicarbonate () 35 mM, calcium (Ca++) 1.5 mM, magnesium (Mg++) 2.0 mM, and potassium (K+) 1.5 mM. All the HDF patients were treated using polysulfone filter (1.3 m2, ultrafiltration coefficient = 42 mL/h mmHg, Hemoflow, F60; Fresenius Medical Care AG, Bad Homburg, Germany). The substitution composition was same as dialysate and was supported using online technique. During HD or HDF, all the patients were anticoagulated with unfractionated heparin using a standard regimen: 24.0 ± 4.4 mg initial bolus followed by infusion of 8 mg/h.
Blood samples were collected from the veins of the limbs opposite to the internal arteriovenous fistulas at the beginning of each dialysis session in the midweek. The waste dialysate samples were collected during the treatments by methods described as fractional direct dialysis quantification (FDDQ) by Charytan et al.Citation13 In the FDDQ method, the ultrafiltration line was disconnected from the effluent line behind the ultrafiltration pump, and the outflow was collected throughout the 4-hour HD session. Then the volume of the fractional collection of dialysate and ultrafiltrate was equal to the volume of ultrafiltrate, and the solute contents in the fractional collection were same as that in the effluent.
The therapeutic regimen of CAPD was 1.5% glucose peritoneal dialysate 2000 mL 3 cycles during day time and 2.5% glucose peritoneal dialysate 2000 mL reserved throughout the night. The urea clearance index (KT/V) was larger than 2.0 per week. Peritoneal dialysate composition was sodium (Na+) 132 mM, lactate 35 mM, calcium (Ca++) 1.77 mM, and magnesium (Mg++) 0.25 mM. Fasting blood samples were collected before the dialysate exchanging in the morning. Then the samples of the mixed spent dialysate in 24 hours were collected.
Fasting blood samples of healthy controls were also collected. Samples of urina sanguinis, from both patients and controls, were collected in the morning when fasting blood samples were collected. Serum samples were separated by blood centrifugation by 1500 × g for 15 minutes. All the samples of serum, waste dialysate, and urine were stored at −80°C until analyzed. Otherwise, the volumes of waste dialysate and urine were recorded when the samples were collected in 1 week. The total removal (TR) of ADMA in 1 week was equal to the sum of the removal by urine and dialysate.
Cleaning dynamics of ADMA by hemodialysis
The TR of ADMA in dialysate was calculated by Eq. (1). Equation (1) was utilized for the samples by the FDDQ method.
where CD, concentration of waste dialysate, that is, mixture solution of dialysate and ultrafiltration; VT, volume of dialysate plus ultrafiltration for a period; VU (mL/min), volume of ultrafiltration; VD, volume of dialysate; QD (mL/min), flow rate of dialysate; Time (min), treatment time.
ADMA determination
Quantification of ADMA was performed on an automated microtiter plate reader (Denley Dragon Well scan MK, Thermo Ltd., Finland) using commercially available enzyme-linked immunosorbent (ELISA) kits (Adlitteram Diagnostic Laboratories, Inc., USA) according to manufacturer's instruction. The minimal detectable concentration for ADMA was 0.01 μg/L.
Glomerular filtration rate detection
To test the residue renal function, GFR was detected by 99m Tc-DTPA (diethylenetriamine pentaacetic acid) two-plasma-sample method of Russell.Citation14
Statistical analysis
Normally distributed variables are given as mean ± SD. Comparisons between groups were performed using unpaired Student's t-test or analysis of variance (ANOVA). A χ2-test was used to compare noncontinuous variables. A two-tailed p-value of 0.05 or less was considered statistically significant. The correlations between ADMA removal and other parameters were tested by linear regression. All calculations were performed using a commercially available program (SPSS for Windows, version 11.0; SPSS, Chicago, Illinois, USA).
RESULTS
The serum levels of ADMA in dialysis patients were much higher than in healthy subjects. For dialysis patients, ADMA levels decreased significantly in PD patients compared with HD or HDF patients (). The 1-week total removal of ADMA in dialysis patients was much lower than in healthy controls (249.21 ± 57.04 μmol/week). For different dialysis groups, the total removal of ADMA decreased significantly by turns of PD, HDF, and HD groups (47.79 ± 8.20 μmol/week, 31.79 ± 8.92 μmol/week, 14.63 ± 6.53 μmol/week, respectively) ().
FIGURE 1. Comparisons of serum ADMA concentrations.
The serum concentrations of ADMA in HC group were much lower than in other groups, **p-values were all less than 0.01. Compared with PD group, the serum ADMA levels in HD and HDF group were both higher, #p < 0.01. There were no significant differences between HD group and HDF group, p > 0.05.
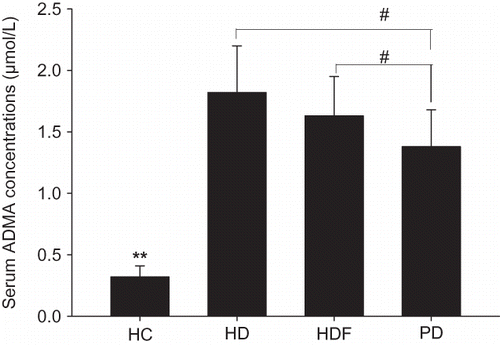
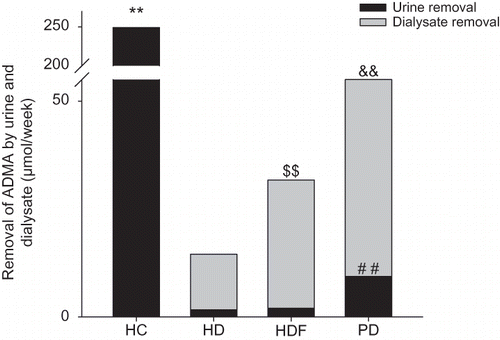
FIGURE 2. Comparisons of ADMA removal by urine and dialysate.
The ADMA removal by urine per week in PD group was highest among dialysis groups, ##p < 0.01. There were no significant differences between HD group and HDF group on urine removal, p > 0.05.
There were significant differences in dialysate removal of ADMA when dialysis groups were compared by turns as PD > HDF > HD. Compared with PD group, the ADMA removals per week through dialysate were much lower in both HDF group and HD group, && with both p-values less than 0.01. Compared with HDF group, the dialysate removal in HD group was reduced significantly, $$p < 0.01.
The total removal of ADMA in HC group, that is, total urine removal, was much higher than in dialysis groups, **p < 0.01. In dialysis groups, the total removal equaled the sum of urine removal and dialysate removal. There were significant differences when HD group, HDF group, and PD group were compared with each other in the sequence of PD>HDF>HD, where the p-values were all less than 0.01.
Removal of ADMA by different dialysis models
The waste dialysate volumes for 1 week were much lower in PD group than in HD or HDF group, and it was less in HD group than in HDF group (). However, it was elevated significantly in PD group on ADMA concentration in waste dialysate. There were significant differences between HD and HDF groups on ADMA concentrations in waste dialysate (). Furthermore, significant differences were found on the calculated 1-week removal of ADMA by dialysate among HD, HDF, and PD groups (12.84 ± 6.69 μmol, 29.60 ± 8.77 μmol, 38.26 ± 8.57 μmol, respectively, p < 0.01) ().
TABLE 2. Parameters of ADMA removal by dialysis
Removal of ADMA by residual renal urine volume
Compared with PD group, the GFR of both HD and HDF groups was reduced significantly. The residual urine volumes for 1 week were much lower in HD or HDF group than in PD group. However, there was no significant difference among HD, HDF, and PD groups on the concentrations of ADMA in urine. Moreover, significant differences were not found between HD and HDF groups on the parameters above (). Then the urine removal of ADMA in 1 week in PD group was higher than in either HD group or HDF group ().
TABLE 3. Parameters of ADMA removal by urine
Correlation factors of ADMA removal
The total removal of ADMA in 1 week was related directly to the spent dialysate concentrations of ADMA (partial regression coefficient, B = 79.49, p < 0.001), the 1-week spent dialysate volume (B = 0.128, p < 0.001), and the 1-week urine volume (B = 0.003, p < 0.001). There were no significant relationships between the total removal of ADMA and the parameters of age, duration of dialysis, GFR, and urinal ADMA level (p > 0.05).
DISCUSSION
CVDs are major contributors to the mortality and morbidity in patients with CKD, and endothelium dysfunction is the most important procedure in the pathogenesis of CVD. According to previous studies, as a uremic toxin, ADMA plays a critical role in endothelium dysfunction. In this study we compared the removal of ADMA by different dialysis therapy methods. The residual urine removals of ADMA were also detected.
Like other previous reports,Citation15—18 it is confirmed again in our study that the serum concentrations of ADMA in maintenence hemodialysis (MHD) patients were significantly higher than those of healthy subjects (). Data from several experimental studies suggest that blood ADMA concentrations are unchanged (4.35 μM vs. 4.76 μM)Citation17 or reduced 23–40% after 4–5 hours HD session,Citation15,Citation16,Citation19 ADMA clearance was 96 ± 6 mL/min, and the total removal in the spent dialysate was 37 μM.Citation17 This study demonstrated that ADMA can be cleaned by dialysis therapy. The total ADMA removals were elevated significantly by turns of HD, HDF, and PD groups (). Both PD and HDF treatment methods can remove ADMA more effectively than HD. This may be because PD and HDF can remove mid–large molecule uremic toxins more effectively. Although ADMA belongs to low-molecular uremic toxin, regular HD cannot remove it effectively, probably due to its high level of binding to plasma proteins.Citation17,Citation20
Clearance of ADMA from the plasma occurs in small part by urinary excretion, but the bulk of ADMA is degraded by intracellular dimethylarginine dimethylaminohydrolase (DDAH), especially in renal tissues, after uptake from the circulation.Citation21,Citation22 In this study, ADMA can be excreted by urine in healthy persons by means of 249 μmol/week. Although the mean GFR was higher in PD group than in HD or HDF group, there were no significant differences on urine ADMA concentrations among three groups. Correlation statistic results show that there were no significant relationships between the total removal of ADMA and the parameters of GFR and urine ADMA level. Then removal of urine ADMA depended on the urine volume in our results. This indicated that residual urine volume is important for clearances of AMDA in ESRD patients. It also suggested that the degradation effect on ADMA by kidney may be tenuity when the patient's GFR was less than 10 mL/min/1.73 m2.
For dialysis patients, the TR of ADMA was primarily achieved through dialysis treatments. Our study indicated that the mean ratios of urine removal to TR of ADMA were for HD group 14.6%, HDF group 7.6%, and PD group 20.4%. Furthermore, the TR of ADMA was related directly to the dialysate ADMA levels, the dialysate volume, and the urine volume. The removal of ADMA by urine and dialysate were both higher in PD patients, so the TR of ADMA was higher and the serum level of ADMA was lower than in other dialysis patients. These results suggest that the methods of selecting different dialysis treatment, increasing exchanged dialysate volume, and conservation of residual urine volume might be helpful in increasing the clearance of ADMA from blood circulation in maintenance dialysis patients. Although there were no correlations between GFR and TR of ADMA in dialysis patients in this study, the residual renal function is very important. Results of Ebinç et al.Citation23 implicated that ADMA levels of PD patients with residual renal function were significantly lower than those without residual renal function (p = 0.01). The conclusion was that residual renal function may lead to a reduction of serum ADMA levels. The ADMA serum levels in PD patients were obviously lower than in HD or HDF patients, which may partly be because the residual renal function was better.
In summary, ADMA was mainly removed by dialysate in dialysis patients. Different dialysis models have different clearance capability on plasma ADMA. PD may be more effective in ADMA removal than HD and HDF, with HDF being more effective than HD.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Morris STW, Mcmurray JJV, Spiers A, Jardine AG. Impaired endothelial function in isolated human uremic resistance arteries. Kidney Int. 2001;60:1077–1082.
- Flecka C, Schweitzera F, Kargea E, Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in patients with chronic kidney diseases. Clinica Chimica Acta. 2003;336:1–12.
- Morris ST, McMurray J, Rodger R, Impaired endothelium-dependent vasodilatation in uraemia. Nephrol Dial Transplant. 2000;15:1194–1200.
- Hand MF, Haynes WG, Webb DJ. Hemodialysis and L-arginine, but not D-arginine, correct renal-failure associated endothelial dysfunction. Kidney Int. 1998;53:1068–1077.
- Kari JA, Donald AE, Vallance DT, Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int. 1997;52:468–472.
- Ravani P, Tripepi G, Malberti F, Asymmetrical dimethyl- arginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. J Am Soc Nephrol. 2005;16:2449–2455.
- Lajer M, Tarnow L, Jorsal A, Plasma concentration of asymmetric dimethylarginine (ADMA) predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2008;31:747–752.
- Zoccali C, Bode B, Mallamaci F, Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet. 2001;358:2113–2117.
- Lu TM, Ding YA, Lin SJ, Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J. 2003;24:1912–1919.
- Schnabel R, Blankenberg S, Lubos E, Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: Results from the AtheroGene study. Circ Res. 2005;97:53–59.
- Valkonen VP, Päivä H, Salonen JT, Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–2128.
- Schulzc F, Lenzen H, Hanefeld C, Asymmetric dimethyl- arginine is an independent risk factor for coronary heart disease: Results from the multicenter Coronary Artery Risk Determination investigating the influnce of ADMA Concentration (CARDIAC) study. Am Heart J. 2006;152(3):493.
- Chaytan C, Gupta B, Meindel N, Fractional directi dialysis quantification: A new approach for prescription and monitoring hemodialysis therapy. Kidney Int. 1996;50(6):1845–1849.
- Russell CD, Bischoff PG, Kontzen FN, Measurement of glomerular filtration rate: Single injection plasma clearance method without urine collection. J Nucl Med. 1985;26(11):1243–1247.
- Anderstam B, Katzatski K, Bergström J. Serum levels of NG, NG-dimethyl-L-arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol. 1997;8:1437–1442.
- Kielstein JT, Böger RH, Bode-Böger SM, Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: Relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol. 1999;10:594–600.
- Kielstein JT, Böger RH, Bode-Böger SM, Low dialysance of asymmetric dimethylarginine (ADMA) – in vivo and in vitro evidence of significant protein binding. Clin. Nephrol. 2004;62(4):295–300.
- Panichi V, Mantuano E, Paoletti S, Effect of simvastatin on plasma asymmetric dimethylarginine concentration in patients with chronic kidney disease. J Nephrol. 2008;21:38–44.
- Wahbi N, Dalton RN, Turner C, Dimethylarginines in chronic renal failure. J Clin Pathol. 2001;54:470–473.
- Böger RH, Zoccali C. ADMA: A novel risk factor that explains excess cardiovascular event rate in patients with end-stage renal disease. Atherosclerosis Suppl. 2003;4:23–28.
- Teerlink T. ADMA metabolism and clearance. Vasc Med. 2005;10(Suppl. 1):S73–S81.
- Carello KA, Whitesall SE, Lloyd MC, Asymmetrical dimethylarginine plasma clearance persists after acute total nephrectomy in rats. Am J Physiol Heart Circ Physiol. 2006;290:H209–H216.
- Ebinç FA, Erten Y, Ebinç H, The relationship among asymmetric dimethylarginine (ADMA) levels, residual renal function, and left ventricular hypertrophy in continuous ambulatory peritoneal dialysis patients. Renal Failure. 2008;30:401–406.