Abstract
This study aimed at evaluating the possible relationship between anorexia and fatigue in hemodialysis (HD) patients and at measuring the plasma levels of interleukin-6 (IL-6) and C-reactive protein (CRP) in HD patients with or without anorexia and/or fatigue. The first question of the Hemodialysis Study Appetite questionnaire was used to assess the appetite of the HD patients and the vitality scale of the SF-36 to assess fatigue. The Charlson Comorbidity Index was assessed in each patient. Seventy-six HD patients were studied. Forty-four were males and 32 females. Thirty-two were classified as not-anorexic and not-fatigued, 12 as not-anorexic but fatigued, 6 as anorexic and not-fatigued, and 26 as anorexic and fatigued. Plasma IL-6 levels (pg/mL) were significantly higher in anorexic and fatigued patients (10.9 ± 11.9) than in not-anorexic and not-fatigued (1.6 ± 0.6) (p < 0.001) and in anorexic but not-fatigued patients (1.8 ± 1.7) (p < 0.01). With respect to not-anorexic but fatigued patients (3.1 ± 1.5), the difference was not statistically significant (p = 0.058). The plasma CRP levels (mg/dL) also were significantly higher in anorexic and fatigued patients (9.2 ± 6.3) than in not-anorexic and not-fatigued patients (4.1 ± 4.5), in anorexic but not-fatigued patients (2.5 ± 1.6), and in not-anorexic but fatigued patients (4.1 ± 4.4) (p = 0.001). The presence of both anorexia and fatigue in chronic HD patients is associated with significantly higher levels of plasma IL-6 and CRP and a higher frequency of comorbidities.
INTRODUCTION
Fatigue, defined as a subjective sense of weakness, lack of energy, and tiredness, is frequent in hemodialysis (HD) patients and significantly impairs their quality of life.Citation1 Depression, anxiety, number and severity of comorbidities, and IL-6 and C-reactive protein (CRP) levels have been shown to be significantly correlated with fatigue in chronic HD patients.Citation2–3 Lee and colleagues have hypothesized that fatigue, considered as a combination of energy exhaustion and decreased mental activity and motivation, is associated with physiological (dialysis duration, hemoglobin, albumin, urea reduction rate, Kt/V), psychological (depression), and situational (gender, age, education, religion, employment) variables.Citation4 Similarly, Jhamb et al. have suggested that in the dialysis population, physiological, behavioral, treatment-related, and individual characteristics may correlate with fatigue.Citation1
In the clinical practice, it is a common observation that anorexia is often associated with fatigue. Anorexia is one of the more common symptoms of chronic HD patients and contributes to poor quality of life.Citation5–6 Its pathogenesis appears complex and only partly known.Citation5–6 Pro-inflammatory cytokines have been suggested as potential mediators of anorexia in HD patients.Citation5–10 However, it is unknown how often both anorexia and fatigue are present in HD patients and which are the demographic, clinical, and laboratory characteristics of HD patients presenting with both these symptoms.
This study aimed at evaluating the possible association between anorexia and fatigue in HD patients, at assessing the frequency of comorbidities and symptoms of depression, and at measuring the plasma levels of interleukin-6 (IL-6) and CRP in the presence of both anorexia and fatigue.
MATERIALS AND METHODS
Patients were recruited from the Catholic University Outpatient Dialysis Clinic. Eligibility requirements were age between 18 and 80 years, thrice weekly in-center HD, and on chronic HD for at least 6 months at study entry. Exclusion criteria were as follows: previous renal transplantation; chronic hepatitis; active malignancy requiring chemotherapy or radiotherapy; active systemic infections such as tuberculosis, sepsis, or systemic fungal infection; chronic inflammatory bowel disease; central venous catheter infection. The study was approved by the local ethics committee and written informed consent was obtained from all patients before enrollment in the study.
Hemodialysis
Patients were maintained on regular HD prescription, three times a week, for 4 hours per session. The blood flow ranged from 250 to 300 mL/min with a dialysis rate flow of 500 mL/min. All patients were treated with high-flux membranes. Most patients were taking antihypertensive medications (β-blockers, calcium-channel blockers, angiotensin-converting enzyme inhibitors) and other commonly used drugs such as phosphate and potassium binders.
Assessment of anorexia and fatigue
The first question of the Hemodialysis (HEMO) Study Appetite questionnaireCitation11 was used to assess the appetite of the HD patients. The multiple-choice answers for the first question “During the past week, how would you rate your appetite?” were as follows: (1) very good, (2) good, (3) fair, (4) poor, (5) very poor. As previously described, patients who rated their appetite very good or good were classified as not-anorexic, whereas the others were defined as anorexic.Citation7
The Italian version of the SF-36 questionnaireCitation12 was offered to the patients by the attending physician. The participants were screened for fatigue status using the vitality scale of the SF-36. The SF-36 vitality scale has good psychometric properties and internal consistency reliability in end-stage renal disease (ESRD) patients.Citation13 Standardized vitality scale scores range from 0 to 100, with higher scores indicating better functioning (i.e., higher levels of energy). Scores above the midpoint of 50 represent well-being (not-fatigued group), whereas scores below 50 represent limitations or disability related to fatigue (fatigued group).
Assessment of comorbidity
Each patient was evaluated for the presence of the following comorbidities included in the Charlson Comorbidity Index and according to the guidelines of the index itself: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, hemiplegia, dementia, chronic obstructive pulmonary disease, peptic ulcer disease, mild liver disease, diabetes without end-organ damage, diabetes with end-organ damage, malignant tumor without metastases (exclude if >5 years from diagnosis), malignant tumor with metastases, acute or chronic leukemia, moderate or severe liver disease, AIDS. The Charlson Comorbidity Index was then calculated in each patient.Citation14–16
The Beck Depression Inventory
We used a validated Italian version of the Beck Depression Inventory (BDI)Citation15 to assess the presence and degree of symptoms of depression. The BDI is a 21-item, patient-rated scale that has been validated in the HD population.Citation16,Citation17 The BDI is a self-administered questionnaire comprising 21 items that evaluates a broad spectrum of depressive symptoms. Of these 21 items, 15 refer to psychological-cognitive symptoms, whereas the remaining 6 items address somatic-vegetative symptoms. Thus, the instrument places greater emphasis on the cognitive component of depression. For each item, the patient is required to select the answer that best reflects his or her present situation and situation in the past week. The total score is obtained by adding the values of the selected phrases, which ranges from 0 to 3. The score range is from 0 to 63 points.
Laboratory measurements
Blood samples were obtained from HD patients directly through the arteriovenous fistula or the central venous catheter immediately before their scheduled HD session at the beginning of the week. The plasma was separated within 30 minutes, and samples were kept frozen at −70°C if not analyzed immediately. Laboratory parameters were measured by routine methods at the Department of Laboratory Medicine, Catholic University of Rome. High-sensitivity CRP (hs-CRP) was measured by means of nephelometry. The limit of detection for plasma hs-CRP was 0.02 mg/dL (0.2 mg/L). Plasma IL-6 was measured by a commercially available photometric enzyme-linked immunosorbent assay (ELISA) (Boehringer Mannheim, Mannheim, Germany).
In each patient, we calculated the weekly erythropoietin (EPO) dose and the EPO resistance index (ERI) defined as the weekly EPO dose divided by Hb level (g/dL). Both the EPO dose and ERI were divided by end-dialysis body weight to indicate the required EPO dose per kilogram of body weight.
Data analysis
Statistical analysis was performed by SYSTAT 7.0 software (SPSS, Chicago, IL, USA). All data were expressed as mean ± SD unless otherwise specified. Box and whiskers plot was used in the figures to describe the distribution of the continuous variables over time. All data were first analyzed for normality of distribution using the Kolmogorov–Smirnov test of normality. The Kruskal–Wallis nonparametric test was used for non-normally distributed continuous variables and analysis of variance was used for normally distributed variables. Categorical variables were compared using the X2-test. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Of the 88 prevalent patients present in the Hemodialysis Service of the Catholic University of the Sacred Heart of Rome, Italy, between July 2008 and August 2009, 5 patients were excluded because of previous renal transplantation, 6 because of chronic hepatitis, and 1 for central venous catheter infection. Of the 76 patients included in the study, 44 were not-anorexic and 32 were anorexic. Not-fatigued and fatigued patients were both 38. The frequency of anorexia was higher among fatigued than among not-fatigued patients (p = 0.03). Nevertheless, the frequency of fatigue was higher among anorexic than among not-anorexic patients (p = 0.03). Accordingly, the mean score of the SF-36 vitality subscale was significantly higher in not-anorexic than in anorexic patients (p = 0.03). Thirty-two patients were classified as notanorexic and not-fatigued, 12 as not-anorexic but fatigued, 6 as anorexic and not-fatigued, and 26 as anorexic and fatigued. Demographic, clinical, and laboratory characteristics of the four groups of patients are shown in .
Table 1. Demographic, clinical, and laboratory characteristics of the four groups of patients
The frequency of comorbid conditions and the Charlson Comorbidity Index in the four groups of patients are shown in . Congestive heart failure, coronary disease, cerebrovascular disease, and peripheral vascular disease were significantly higher in anorexic and fatigued patients than in not-anorexic and not-fatigued ones. The Charlson Comorbidity Index was significantly higher in anorexic and fatigued (8.2 ± 1.4), than in anorexic but not-fatigued (5.1 ± 2.3), not-anorexic and fatigued (2.8 ± 1.8), and not-anorexic and not-fatigued (2.2 ± 1.4) patients.
Table 2. Comorbidity and Charlson Comorbidity Index
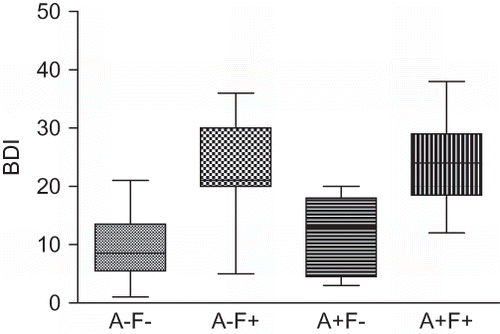
Considering all patients included in the study, the mean (±SD) BDI score was 15.9 ± 9.4. The BDI score was significantly higher in anorexic and fatigued patients than in not-anorexic and not-fatigued ones and in patients who presented with only anorexia. The BDI was also significantly higher in not-anorexic but fatigued patients than in not-anorexic and not-fatigued ones ().
Figure 1. BDI in not-anorexic and not-fatigued (A–F−), not-anorexic but fatigued (A–F+), anorexic and not-fatigued (A+F−), and anorexic and fatigued (A+F+) patients. A+F+ versus A–F−, p < 0.001; A+F+ versus A+F−, p < 0.05; A+F+ versus A–F+, p < 0.001.
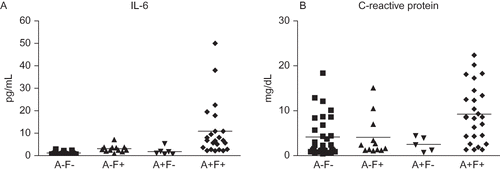
As detailed in the , plasma IL-6 levels (pg/mL) resulted significantly higher in anorexic and fatigued patients (10.9 ± 11.9) than in not-anorexic and not-fatigued (1.6 ± 0.6) (p < 0.001) and in anorexic but not-fatigued patients (1.8 ± 1.7) (p < 0.01). With respect to not-anorexic but fatigued patients (3.1 ± 1.5), the difference was not statistically significant (p = 0.058). The plasma CRP levels (mg/dL) also were significantly higher in anorexic and fatigued patients (9.2 ± 6.3) than in not-anorexic and not-fatigued patients (4.1 ± 4.5; p < 0.01), anorexic but not-fatigued patients (2.5 ± 1.6; p < 0.01), and not-anorexic but fatigued patients (4.1 ± 4.4; p < 0.01) ().
Figure 2. Interleukin-6 (Panel A) and C-reactive protein (Panel B) levels in not-anorexic and not-fatigued (A−F−), not-anorexic but fatigued (A−F+), anorexic and not-fatigued (A+F−), and anorexic and fatigued (A+F+) patients. Interleukin-6: A+F+ versus A−F−, p < 0.001; A+F+ versus A+F−, p < 0.01; A+F+ versus A−F+, p = 0.058. C-reactive protein: A+F+ versus other groups, p = 0.001. Other differences are not statistically significant.
DISCUSSION
This study shows that plasma IL-6 and CRP levels are significantly higher in HD patients who are both anorexic and fatigued than in patients who do not complain anorexia and fatigue or suffer only one of such symptoms. To our knowledge, this is the first study that reports such results.
Indeed, the pathogenesis of both anorexia and fatigue in HD patients is only partially known.Citation1–8 An association also between anorexia and high levels of pro-inflammatory cytokines has been recently documented in two large studies.Citation4–6 It is well known that cytokines are able to inhibit appetite both in healthy conditions and in various diseases (cancer, sepsis, cardiac cachexia, chronic obstructive pulmonary disease).Citation18–22 Thus, it may be questioned why, in this study, the IL-6 levels of patients who had only anorexia did not differ from those of patients who complained fatigue alone or of patients who were both not-anorexic and fatigued. The answer to this question is difficult but some considerations may be helpful: (1) in a recent study we found a trend toward higher levels of CRP in patients with poor or very poor appetite with respect to patients with very good or good appetite, although the differences were not statistically significant;Citation8 (2) in this study, the median IL-6 levels in patients without anorexia and fatigue, with anorexia alone, and with fatigue alone were particularly low (all below 3 pg/mL); (3) our patients, unlike those of other studies,Citation4,Citation5 received a Mediterranean diet and this may have contributed to low levels of IL-6;Citation23 (4) we did not compare IL-6 patients according to the sex and the appetite although it has been suggested that HD women are less susceptible to the inflammation-induced anorexia.
Cytokines have been involved also in the pathogenesis of fatigue.Citation1 Cytokines may cause fatigue through direct activation of the central nervous system, hypothalamus, pituitary gland, and adrenal glands or creating a status of chronic inflammation or indirectly by inducing sleep disorders, depression, or anxiety.Citation1 We have recently shown that fatigue was correlated with age, dialytic age, number and severity of comorbidities, symptoms of depression and anxiety, and albumin and IL-6 levels in a group of chronic HD patients.Citation10 Nevertheless, there is evidence of a significant correlation between fatigue and circulating levels of IL-6 in patients with other diseases such as cancer, polycystic ovary syndrome, psoriasis, rheumatoid arthritis, and the geriatric syndrome of frailty.Citation24–28
Another finding of our study is that the Charlson Comorbidity Index and the BDI score were significantly higher in patients with both anorexia and fatigue than in other groups of patients. This result might be expected but, however, it is interesting to note that, to our knowledge, it is the first time that it has been demonstrated in HD patients. Indeed, comorbid conditions imply stress, emotions, waiting for diagnostic procedures, time spent in ambulatory and/or day hospital, and new therapies or therapy switches, and it is possible that all these conditions may imply tiredness, exhaustion, concern, and, ultimately, fatigue and disturbances of appetite.
We also found that more than 40% of patients, in this study, were anorexic and that of these most (81.2%) were also fatigued. Conversely, among the fatigued patients, which were 50% of the study population, those who were also anorexic were 68.4%. In general, symptoms in ESRD are under-recognized. Prevalence studies have focused on single symptom rather than on more symptoms or on the whole range of symptoms experienced. Recently, a systematic review showed that fatigue and anorexia had high prevalence, 71% and 49%, respectively.Citation29 Similarly to what happens in cancer patients,Citation30,Citation31 it has also been shown that incident HD and peritoneal dialysis patients experience concurrent symptoms.Citation31 In particular, it has been shown that three distinct clusters of symptoms could be derived in HD patients. Cluster 1 included symptoms such as shortness of breath, feeling faint/dizzy, poor appetite, feeling “squeezed out,” and feeling nauseous; the second cluster consisted of symptoms such as sore muscles, chest pain, and numbness in the hands/feet; whereas the symptoms of itch and dry skin made up cluster 3, which could reflect an underlying dimension of “skin problems.” Surprisingly, none of the three clusters included fatigue.Citation31 Our findings suggest that anorexia and fatigue in HD patients are strongly associated with very high rate of prevalence. It remains to clarify if anorexic HD patients become fatigued with time or vice versa and if this knowledge would be worthwhile to prevent such possible progression. In this regard, it seems that adequate longitudinal studies are needed.
This study has certain limitations. First and foremost, our patient population was quite small and drawn from a single geographic area. However, it must be considered that many patients with acute or chronic infections, malignant tumors, or previous history of renal transplantation were excluded to avoid interferences with IL-6 and CRP measurements. Second, the assessment of fatigue was made through the vitality scale of the SF-36 questionnaire, an instrument that measures the experience of fatigue during a period ranging from weeks to months and may fail to recognize daily or weekly fatigue fluctuations. The SF-36 fatigue subscale is the most used tool to assess fatigue in ESRD patients receiving chronic dialysisCitation1,Citation3,Citation32,Citation33 and has been used also with people with chronic diseases such as obstructive pulmonary disease, Sjogren syndrome, spinal cord injury, and systemic lupus erythematosus.Citation3,Citation35–37
In summary, the presence of both anorexia and fatigue in chronic HD patients is associated with significantly higher levels of plasma IL-6 and CRP and a higher frequency of comorbidities. These findings should help to generate additional longitudinal studies to possibly demonstrate the causative role of chronic high levels of cytokines and, in particular, IL-6 in the onset of fatigue and/or anorexia in HD patients.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Jhamb M, Weisbord SD, Steel JL, Unruh M. Fatigue in patients receiving maintenance dialysis: A review of definitions, measures, and contributing factor. Am J Kidney Dis. 2008;52: 353–365.
- Bossola M, Luciani, T. Fatigue and its correlates in chronic hemodialysis patients. Blood Purification. 2009;28:245–252.
- Jhamb M, Argyropoulos C, Steel JL, Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) study. Clin J Am Soc Nephrol. 2009;4:1779–1786.
- Lee BO, Lin CC, Chaboyer W, Chiang CL, Hung CC. The fatigue experience of hemodialysis patients in Taiwan. J Clin Nurs. 2007;16:407–413.
- Bossola M, Tazza L, Giungi S, Luciani G. Anorexia in hemodialysis patients: An update. Kidney Int. 2006;70:417–422.
- Carrero JJ, Qureshi AR, Axelsson J, Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am J Clin Nutr. 2007;85:695–701.
- Kalantar-Zadeh K, Block G, McAllister CJ, Appetite and inflammation, nutrition, anemia, and clinical outcome in HD patients. Am J Clin Nutr. 2004;80:299–307.
- Burrowes JD, Larive B, Chertow GM, Self-reported appetite, hospitalization and death in HD patients: Findings from the HD [HEMO] Study. Nephrol Dial Transplant. 2005;20: 2765–2774.
- Bossola M, Scribano D, Colacicco L, Anorexia and plasma levels of free tryptophan, branched chain amino acids, and ghrelin in hemodialysis patients. J Ren Nutr. 2009;19(2): 48–55.
- Bossola M, Giungi S, Luciani G, Tazza L. Appetite in chronic hemodialysis patients: A longitudinal study. J Ren Nutr. 2009;19:372–379.
- Lopes AA, Elder SJ, Ginsberg N, Lack of appetite in hemodialysis patients – associations with patient characteristics, indicators of nutritional status and outcomes in the international DOPPS. Nephrol Dial Transplant. 2007;22: 3538–3546.
- Mingardi G, Cornalba L, Cortinovis E, Ruggiata R, Mosconi P, Apolone G. Health-related quality of life in dialysis patients. A report from an Italian study using the SF-36 Health Survey. DIA-QOL Group. Nephrol Dial Transplant. 1999;14:1503–1510.
- Wu AW, Fink NE, Cagney KA, Developing a health-related quality-of-life measure for end-stage renal disease: The CHOICE Health Experience Questionnaire. Am J Kidney Dis. 2001;37:11–21.
- Di Iorio B, Cillo N, Cirillo M, De Santo NG. Charlson Comorbidity Index is a predictor of outcomes in incident hemodialysis patients and correlates with phase angle and hospitalization. Int J Artif Organs. 2004;27:330–336.
- Sica C, Ghisi M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In: Lange MA, ed., Leading-Edge Psychological Tests and Testing Research. Hauppauge, New York: NOVA Publishers; 2007:27–50.
- Chilcot J, Wellsted D, Farrington K. Screening for depression while patients dialyse: An evaluation. Nephrol Dial Transplant. 2008;23:2653–2659.
- Watnick S, Wang PL, Demadura T, Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis. 2005;46(5):919–924.
- Kim HR, Son GR. Fatigue and its related factors in Korean patients on hemodialysis. Taehan Kanho Hakhoe Chi. 2005;35: 701–708.
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899.
- Langhans W. Signals generating anorexia during acute illness. Proc Nutr Soc. 2007;66:321–330.
- Teli T, Xanthaki D, Karalis KP. Regulation of appetite and insulin signaling in inflammatory states. Ann N Y Acad Sci. 2006;1083:319–328.
- Wong S, Pinkney J. Role of cytokines in regulating feeding behaviour. Curr Drug Targets. 2004;5:251–263.
- Giugliano D, Ceriello A, Esposito K. The effect of diet on inflammation. Emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–685.
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav Immun. 2007;21:413–427.
- Vgontzas AN, Trakada G, Bixler EO, Plasma interleukin 6 levels are elevated in polycystic ovary syndrome independently of obesity or sleep apnea. Metabolism 2006;55: 1076–1082.
- Yasumoto S, Imayama S, Hori Y. Increased serum level of interleukin-6 in patients with psoriatic arthritis and thrombocytosis. J Dermatol. 1995;22:718–722.
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: Implications for fatigue. Brain Behav Immun. 2008;22:24–32.
- Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: A pilot study. J Am Geriatr Soc. 2002;50: 1268–1271.
- Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis. 2007;14:82–99.
- Walsh D, Rybicki L. Symptom clustering in advanced cancer. Support Care Cancer. 2006;14:831–836.
- Yennurajalingam S, Palmer JL, Zhang T, Poulter V, Bruera E. Association between fatigue and other cancer-related symptoms in patients with advanced cancer. Support Care Cancer 2008;6:1125–1130.
- Thong MSY, van Dijk S, Noordzij M, NECOSAD Study Group. Nephrol Dial Transplant. 2008;24:225–230.
- Whitehead L. The measurement of fatigue in chronic illness: A systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. 2009;37:107–128.
- Gift AG, Shepard CE. Fatigue and other symptoms in patients with chronic obstructive pulmonary disease: Do women and men differ? J Obstet Gynecol Neonatal Nurs. 1999;28:201–208.
- Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Measurement of fatigue in systemic lupus erythematosus: A systematic review. Arthritis Rheum. 2007;57:1348–1357.
- Strömbeck B, Theander E, Jacobsson LT. Assessment of fatigue in primary Sjögren's syndrome: The Swedish version of the Profile of Fatigue. Scand J Rheumatol. 2005;34: 455–459.
- Anton HA, Miller WC, Townson AF. Measuring fatigue in persons with spinal cord injury. Arch Phys Med Rehabil. 2008;89:538–542.