Abstract
Background: Bone marrow cell has been proposed as a source of new mesothelium, but supporting evidence is rare. This study examines the validity of this hypothesis by using green fluorescent protein (GFP) and Y-chromosome trackers to identify the presence of donor marrow cells in peritoneal membrane of bone marrow transplant recipient mice. Methods: Cross-gender and GFP-mismatched bone marrow transplantation was undertaken in 20 FVB mice. Five recipients were killed 2, 4, and 6 weeks and 6 months later. Peritoneal tissues were obtained for the detection of GFP and Y chromosome by immunohistochemical staining (IHC) and chromogenic in situ hybridization (CISH). Results: GFP+ cells could be found in the peritoneal membrane of bone marrow transplant recipients. However, the level of engraftment was low, accounting for 0.9%, 0.8%, 0.7%, and 2.2% of the total counted mesothelial cells in intestinal serosa at 2, 4, and 6 weeks and 6 months post-transplantation, respectively. The presence of donor marrow cells within mesothelium was again confirmed by the detection of Y-chromosome-containing cells. Moreover, Y-chromosome+ cells incorporated within the mesothelium were positively stained by anticytokeratin antibody. Conclusions: Donor marrow cells could attach to mesothelium and exhibit mesothelial marker cytokeratin in bone marrow transplant recipients. This finding suggests that bone marrow-derived cells might participate in the turnover of mesothelium.
INTRODUCTION
The exact mechanism of mesothelial repair remains controversial. Healing of the mesothelium was previously thought to be achieved by centripetal migration from the damaged area as the healing process of skin.Citation1 However, experimental observations revealed that the entire wound surface became epithelialized simultaneously rather than gradually from the border. In addition, large peritoneal wounds healed at the same speed as a small one. Thus, proliferation and centripetal migration of cells from the wound edge could not explain the whole mechanism of mesothelial repair. Additional sources of mesothelial progenitor cells should exist.Citation2–8 Several hypotheses have been proposed regarding the source of new mesothelium.
The first hypothesis proposed that a new mesothelium is formed by the transformation of multipotent submesothelial cells, which migrate upward from the base of mesothelial wound. The hypothesis was proposed based on the findings that repair process of mesothelium takes place simultaneously and rapidly over the entire wound.Citation2–4,Citation6 Additionally, subserosal cells were positively stained by cytokeratin in the inflamed healing serosa, suggesting that submesothelial cells might transdifferentiate into mesothelial phenotype.Citation9
The second hypothesis proposed that a new mesothelium is formed from peritoneal free-floating populations. Cameron and colleaguesCitation10 described that islands of cells appeared on wound surfaces within 24 h of mesothelium injury. These cells spread out from the islands and formed a new sheet of mesothelium. Johnson et al.Citation11 reported that peritoneal wound healing was considerably delayed if the wound was covered with polythene. In contrast, there was a rapid seeding of cells on the free surface of the wound and polythene. These attached cells soon formed a continuous mesothelial layer. Subsequent evidenceCitation5,Citation7,Citation10–16 also strongly supports the presence of peritoneal free-floating progenitors and these cells were believed to come from exfoliative mesothelial cells.Citation7,Citation17–19
The third hypothesis proposed that circulating bone marrow cells are an alternative source of mesothelial progenitors. Previous investigators Johnson et al.Citation11 put forward the idea that a new mesothelium might come from monocytes under special circumstances. Wagners et al.Citation20 also hypothesized that the mononuclear population implicated in the repair of mesothelium might be primitive stem cells originating from bone marrow. However, supporting evidence was lacking. Recently, several investigators reported findings in support of this hypothesis. Takanobu et al.Citation21,Citation22 showed that exogenous bone marrow mononuclear cells infused into peritoneal cavity of mice could attach to the peritoneal membrane and differentiate into cytokeratin-positive cells. SekiguchiCitation23 revealed that c-kit+ cells (a marker of immature bone marrow-derived cells) could be detected in the peritoneal membrane during the course of chlorhexidine-induced peritoneal injury. Lucas et al.Citation24 reported that intraperitoneal injection of mesenchymal stem cells (MSC) could reduce peritoneal adhesion caused by peritoneal injury. They postulated that MSC had the capacity to differentiate into mesothelial cells and repair the injured mesothelium.
Bone marrow cells possess wide range of plasticity. They can differentiate into many types of adult cells both in vivo and in vitro. The highly plastic marrow cells might play a role in the maintenance of several mesodermal tissues.Citation25–29 Embryologically, both mesothelium and bone marrow arise from the mesoderm.Citation30 It is thus reasonable to postulate that bone marrow cells might transdifferentiate into mesothelial cells and participate in the mesothelial remodeling.
In this work, we examined the possibility of bone marrow cells as a source of new mesothelium by using both green fluorescent protein (GFP) and Y-chromosome trackers to trace the donor marrow cells in the mesothelium of bone marrow transplant recipients.
METHODS
Design
Following bone marrow ablation with a lethal-dose irradiation, 20 female wild-type mice were injected with whole bone marrow cells obtained from male GFP transgenic donors. Then, groups of five recipients were killed at 2, 4, and 6 weeks and 6 months after bone marrow transplantation. Peritoneal tissues were obtained at the time of killing to detect the GFP- and Y-chromosome-containing cells. Combined Y-chromosome in situ hybridization and cytokeratin immunohistochemical staining was used to determine whether the donor marrow cells could express the mesothelial phenotype when incorporated into the mesothelial layer.
Animals
All animal procedures were carried out in accordance with the protocol approved by the institutional committee for animal research of Keelung Chang Gung Memorial Hospital and the ethical guidelines of the National Research Council of USA. Wild-type FVB mice were purchased from the National Laboratory Animal Center, Taipei, Taiwan. GFP transgenic mice (FBV/N) were obtained from Level Biotechnology Inc., Taipei, Taiwan.Citation31
Bone marrow transplantation
Male transgenic mice that ubiquitously express GFP were used as the donors. To harvest bone marrow cells, animals were killed by cervical dislocation, and then their femurs and tibias were removed. Using a syringe and a 23-gauge needle, the bone marrow cells were flushed into a sterile 4 mL tube. After red blood cell (RBC) lysis and sieving through a 50 μm mesh, the cells were washed, counted, and used for transplantation.
Eight-week-old female wild-type mice were used as the recipients. Each recipient animal received whole-body gamma-ray irradiation at a dose of 900 rads. Unfractionated bone marrow cells (5 × 106 nucleated cells in 50 μL PBS) obtained from donor mice were injected into the tail vein of recipients within 24 h of irradiation.
Treatment of tissue sections
Mesothelium-covered organs, including the liver, intestine, mesentery, pancreas, and anterior abdominal wall, were harvested at the time of killing. Harvested organs were initially placed in 4% paraformaldehyde/10% sucrose in PBS at 4°C to hold GFP protein within the cytoplasm. Then, these organs were fixed overnight in 10% neutral buffered formalin and embedded in paraffin wax. Sections of 4 μm thickness were obtained for both immunohistochemical staining (IHC) and chromogenic in situ hybridization (CISH).
Immunohistochemical staining
Paraffin-embedded sections were dewaxed, incubated with 3% hydrogen peroxide in methanol to quench endogenous peroxidase activity, and taken through a graded series of alcohols to PBS. Antigen retrieval was achieved by heating in pressure cooker with 10 mM citrate buffer (pH 6.0) at 100°C for 20 min. Nonspecific immunoglobulin-binding sites were blocked using nonimmune goat serum.
For the demonstration of GFP protein in the mouse tissue, slides were incubated with rabbit anti-GFP antibody (Chemicon, Temecula, CA, USA) at 4°C overnight at a dilution of 1:400. Sections were then washed in PBS and incubated with a biotinylated secondary antibody–streptavidin–horseradish peroxidase (HRP) system (DAKO, Carpinteria, CA, USA) for 60 min at room temperature. Following washes in PBS, enzymatic detection was performed with the application of diaminobenzidine (DAB) substrate (DAKO) for 10 min at room temperature.
Chromogenic in situ hybridization
For the demonstration of Y chromosome, sections were incubated in 1 M sodium thiocyanate at 80°C for 10 min, washed in PBS, and then digested in 0.4% pepsin in 0.1 M HCl at 37°C for 10 min. The reaction was quenched in 0.2% glycine in 2×PBS and the sections were then rinsed in PBS, post-fixed in 4% paraformaldehyde in PBS, dehydrated through graded alcohols, and air-dried.
A FITC-conjugated mouse Y-chromosome probe (Star-FISH, Cambio, Cambridge, UK) was then added to the center of each tissue section and covered with a coverslip of suitable size. The edges were sealed with a thin layer of rubber cement to prevent the evaporation of probe solution during incubation. Hybridization was performed by heating the slides to 60°C for 10 min and incubating at 37°C for 16–18 h. After removing the rubber cement and coverslip, the slides were washed in 50% formamide/2×SSC at 37°C, then washed with 2×SSC, incubated with 4×SSC/0.05% Tween/5% milk powder for 10 min at 37°C. After three washes of 2 min each with PBS, endogenous biotin blocking was performed with casein blocking solution. The slides were incubated with 1:3000 peroxidase-conjugated rabbit polyclonal anti-fluorescein (Abcam, Cambridge, UK) for 60 min at room temperature. Signal detection was performed using DAB (DAKO) as the chromogen. The slides were counterstained with hematoxylin, cleared in xylene, and then mounted with Permount (Fisher Scientific, Loughborough, UK).
Combined immunohistochemistry and chromogenic in situ hybridization analysis
Tissue sections were sequentially stained for mesothelial marker (cytokeratin staining by the IHC method) to identify cells of a mesothelial phenotype, followed by Y-chromosome in situ hybridization to identify cells of donor bone marrow origin.Citation32,Citation33
Step 1: cytokeratin immunohistochemistry
Four-micrometer sections were dewaxed. Their endogenous peroxidases were blocked with 3% hydrogen peroxide in methanol, and alkaline phosphatase activity was blocked with acetic acid in methanol. Then, tissues were taken through graded alcohols to PBS. Slides were incubated with rabbit anti-pancytokeratin primary antibody (DAKO) at room temperature for 1 h, followed by biotinylated secondary antibody. A tertiary layer of streptavidin–alkaline phosphatase (Vector Laboratories, Peterborough, UK) in PBS was applied. Color was then developed using Vector Red Substrate (Vector Laboratories) for 15 min at room temperature. The sections were washed in PBS prior to in situ hybridization protocol.
Step 2: in situ hybridization for Y chromosome
Cytokeratin-stained sections were then processed as the procedure described above for “chromogenic in situ hybridization.”
Flowcytometry
The presence of GFP-expressing cells in blood or bone marrow was examined by flowcytometric analysis to determine whether donor marrow cells successfully engrafted and repopulated the bone marrow of recipients.
In brief, blood and bone marrow obtained from wild-type mice, GFP donors, or transplant recipients were treated with RBC lysing solution, washed with PBS, and then analyzed with a FAC scan instrument (Beckman Coulter, Fullerton, CA, USA) at a cell concentration of 1 × 106 cells/mL.
Using forward- and side-scatter parameters, dead cells and debris were eliminated from the analysis (mainly in the blood sample) to obtain a histogram of the distribution of GFP-expressing cells. GFP was excited by an argon laser and fluorescence was detected using a 530/30 nm bandpass filter in the FL1 channel.
RESULTS
Efficacy of bone marrow transplantation
Flowcytometric analysis revealed that most of the mononuclear cells in the blood (after gating to exclude dead cells and the debris of RBC) and bone marrow of all recipients expressed green fluorescence. The percentage of GFP+ cells in peripheral blood and bone marrow of recipients was 80% and 95%, respectively. This finding indicated that bone marrow transplantation was successful and that the bone marrow of the recipient was reconstituted with donor marrow cells.
Anti-GFP immunohistochemical staining
In GFP transgenic mice, most mesothelial cells (approximately 82%) were positively stained with anti-GFP antibody (A); however, no mesothelial cells of the wild-type mice (0%) were positive for anti-GFP antibody staining (B).
Figure 1. (A) Immunohistochemical staining of GFP revealed that cells in intestinal serosa (arrow), epithelium of intestinal villi (star), and stroma (hollow arrowhead) were positively stained with anti-GFP antibody in GFP transgenic mice. (B) No cells were stained by anti-GFP antibody in wild type mice. (C–E) GFP+ cell (arrow) could be identified in the parietal peritoneum of the anterior abdominal wall (C), the visceral peritoneum of the liver (D), and the intestinal serosa (E) of the transplant recipients, many infiltrating leukocyte (hollow arrowhead, 1E) in the stroma of intestinal villi were also positive for anti-GFP. (F) Percentage of anti-GFP+ cells within intestinal serosa of GFP donors, wild-type mice, and transplant recipients sacrificed at different time.
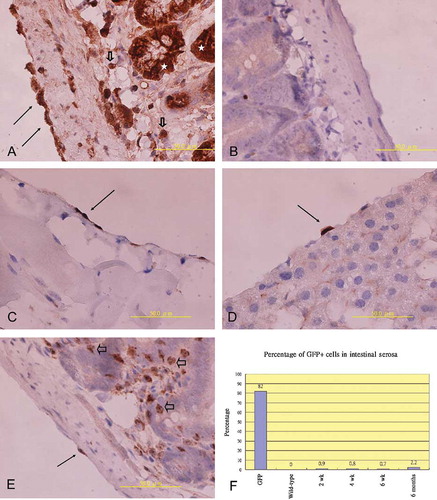
In bone marrow transplant recipients, GFP+ cells could be detected within the mesothelial layer of the anterior abdominal wall (C), liver (D), intestine (E), and mesentery (not shown). However, the number of GFP+ cells within mesothelial layer of recipients was very small, accounting for 0.9% (40/4493), 0.8% (31/3677), 0.7% (22/3247), and 2.2% (164/6013) of the total counted mesothelial cells in the intestinal serosa of recipients killed at 2, 4, and 6 weeks and 6 months after bone marrow transplantations, respectively (F). Most GFP+ cells within the mesothelial layer exhibited a cuboidal to squamous shape resembling the morphology of mesothelial cells (C–E).
Y-chromosome chromogenic in situ hybridization
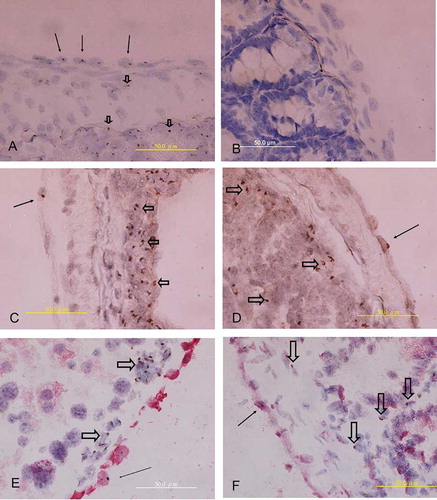
In a section of 4 μm thickness, Y chromosome was present only in parts of the peritoneal mesothelial cells. The detection rate of Y chromosome in the mesothelial cells of male mice was between 30% and 50% (A, arrow), and zero in female negative controls (B). Given that the detection rate of Y-chromosome+ cells was low in mesothelium and peritoneal membrane was frequently damaged following pepsin treatment, Y-chromosome marker positive cells were very rare in the mesothelium of recipients (C and , arrow). In contrast, Y-chromosome+ donor cells were frequently found in the stroma of intestine (hollow arrowhead, C, ). Because of these limitations, the detection rate of Y-chromosome+ cells within mesothelium was not calculated. The identification of Y-chromosome+ cells within mesothelium was used only to confirm the findings of anti-GFP immunostaining.
Figure 2. (A) Y-chromosome+ cells were identified in serosa (arrows) as well as in stroma (hollow arrowhead) of intestine in male positive control. (B) No Y-chromosome+ cells were found in female negative control. (C and D) A few Y-chromosome+ cells (arrow) were found on intestinal serosa of recipients. Alternatively, plenty of Y-chromosome+ leukocyte (hollow arrowhead) existed in the stroma of intestine. (E) Y-chromosome+ cell (brown color, arrow) in peritoneum of the liver also expressed cytokeratin marker (red color); however, Y-chromosome+ leukocyte (hollow arrowhead) in submesothelial area were negative for cytokeratin staining. (F) Y-chromosome+ cell (arrow) in intestinal serosa of recipient was also positive for cytokeratin staining (weakly staining of Vector Red following pepsin treatment in the in situ hybridization procedure), however, several Y-chromosome+ leukocyte (hollow arrowhead) in stroma were negative for cytokeratin.
Combined cytokeratin immunostaining and Y-chromosome in situ hybridization
A double staining technique that combined Y-chromosome CISH and cytokeratin IHC revealed that some Y-chromosome+ marrow cells located within mesothelium also expressed the mesothelial marker cytokeratin. As Y-chromosome+ cells were rarely found in mesothelium, it was difficult to detect cells that co-expressed the Y chromosome and cytokeratin in the mesothelial layer. Only a few such cells were identified in this study (E, , arrow). Alternatively, Y-chromosome+ cells within stroma or connective tissue were not positive for cytokeratin (hollow arrowhead, E, ).
DISCUSSION
The results of this study showed that donor marrow cells could attach to peritoneal membrane, turn into mesothelial cell morphology, and express cytokeratin antigen in bone marrow transplant recipient mice. These findings support the postulation that bone marrow cells could be a source of mesothelial progenitors.
Our findings are consistent with those of Takanobu et al.Citation21,Citation22 and Kaneko group,Citation23 however, contradictory to those of Ito et al.Citation34 Takanobu et al.Citation21,Citation22 reported that IP-infused bone marrow cells could attach to the peritoneal membrane and differentiate into cytokeratin-positive cells in rat. Furthermore, this intervention improved the recovery of peritoneal function and reduced peritoneal fibrosis. SekiguchiCitation23 found that cells of bone marrow origin (c-kit+ cells) could be detected in peritoneal membrane during the course of peritoneal injury caused by chlorhexidine gluconate in mice. However, Ito et al.Citation34 showed that no GFP+ cells differentiated into new mesothelium in a GFP-mismatched bone marrow transplantation model in rats. The discrepancy in the findings between different authors suggests that further experiments are necessary to delineate the exact answer. If this hypothesis could be confirmed in future studies, transplantation of bone marrow cells might be a potential treatment to repair the injured peritoneal membrane.
Whitaker et al.Citation7 denounced the possibility of bone marrow cells to be mesothelial progenitors based on the observation that mesothelial healing was not impaired after whole-body X-irradiation to deplete bone marrow stem cells. In fact, depletion of bone marrow cells by irradiation might not retard mesothelial repair if bone marrow cells play only a very minor role in mesothelial remodeling. Thus, the findings of Whitaker et al. could not reject the possibility of bone marrow cells to be an alternative source of mesothelial progenitors.
As no acute peritoneal injury was caused in this study, the result of this experiment can be regarded as a model to examine the role of bone marrow cells in the normal turnover of mesothelium. The presence of GFP+ cells in the peritoneum of recipients 2, 4, and 6 weeks or 6 months after transplantation suggests that bone marrow cells might participate in the normal turnover of mesothelium.
Most data regarding the contribution of bone marrow cells in organ repair come from studies on specific organ injury.Citation32,Citation35,Citation36 These data revealed that bone marrow cells usually contribute little to the normal turnover of tissues, but that their roles in tissue repair become significant under circumstances of acute injury. As we have encountered technical difficulty in causing acute peritoneal injury in small mice, such intervention was not undertaken in this study. If future acute peritoneal injury studies reveal the presence of more abundant bone marrow cells in mesothelial layer, the role of bone marrow cells in peritoneal repair can be further confirmed.
Bone marrow contains several types of stem cells, such as MSC, hematopoietic stem cells (HSC), and endothelial progenitor cells (EPC). Of these, pluripotent MSC can reconstitute multiple tissues and organs of mesodermal origin. Previous in vitro cell culture studies had shown that the primitive mesenchyme could differentiate into mesothelial cells.Citation37 Together with the finding of Lucas et al.Citation24 who showed that MSC could reduce adhesion caused by peritoneal injury, we believe that MSC is the fraction of bone marrow cells that participates in mesothelial turnover. HSC is mainly responsible for the maintenance of hematopoietic cell lineages and is unlikely to be involved in the reconstitution of mesothelium. However, a few investigators showed that HSC might also contribute to the repair of nonhematopoietic tissue.Citation25,Citation28 Thus, the possibility of HSC to be a source of new mesothelial cell cannot be completely excluded. A transformation process that converts monocytes or macrophages to a mesothelial phenotype has been proposed by previous investigators.Citation5,Citation11,Citation13 Nevertheless, no laboratory evidence could support the presence of such a transformation process.
The result of the current study is very preliminary. Future in vivo acute peritoneal injury studies and in vitro coculture experiments are needed. Furthermore, co-staining of Y chromosome with more mesothelial markers is required to confirm the role of bone marrow cells in mesothelial turnover. In brief, our results provide weak but new evidence in support of the hypothesis that bone marrow-derived cells might be an alternative source of new mesothelium, although at a very low level.
Acknowledgment
This work was supported by grants from the Keelung Chang Gang Memorial Hospital (CMRPG250011).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Cunningham RS. The physiology of the serous membrane. Physiol Rev. 1926;6:242–280.
- Robbins GF, Brunschwig A, Foote FW. Deperitonealization: Clinical and experimental observations. Ann Surg. 1949;130: 466–479.
- Williams DC. The peritoneum. A plea for a change in attitude towards this membrane. Br J Surg. 1955;42:401–405.
- Ellis H, Harrison W, Hugh TB. The healing of peritoneum under normal and pathological conditions. Br J Surg. 1965;52: 471–476.
- Eskeland G, Kjaerheim A. Regeneration of parietal peritoneum in rats. 2. An electromicroscopical study. Acta Pathol Microbiol Scand. 1966;68:379–395.
- Raftery AT. Regeneration of parietal and visceral peritoneum: An electron microscopical study. J Anat. 1973;115:375–392.
- Whitaker D, Papadimitriou J. Mesothelial healing: Morphological and kinetic investigations. J Pathol. 1985;145:159–175.
- Mutaaers SE, Whitker D, Papadinitriou JM. Mesothelial regeneration is not dependent on subserosal cells. J Pathol. 2000;190:86–92.
- Bolen JW, Hammer SP, McNutt MA. Reactive and neoplastic serosal tissue. Am J Surg Pathol. 1986;10:34–47.
- Cameron GR, Hassan SM, De SN. Repair of Glisson's capsule after tangential wounds of the liver. J Pathol Bacteriol. 1957; 73:1–10.
- Johnson FR, Whitting HW. Repair of parietal peritoneum. Br J Surg. 1962;49:653–660.
- Eskeland G, Kjaerheim A. Growth of autologous peritoneal fluid cells in intraperitoneal diffusion chamber in rats. II An electron microscopical study. Acta Pathol Microbiol Scand. 1966;68:501–516.
- Ryan GB, Grobety J, Majno G. Mesothelial injury and recovery. Am J Pathol. 1973;71:93–112.
- Venables BC, Ellis H, Burns JE. The effect of X radiation on peritoneal healing: An experimental study. Br J Radiol. 1967;40:275–279.
- Cleaver CLT, Hopkins AD, Kwong K, Raftery AT. The effect of postoperative peritoneal lavage on survival, peritoneal wound healing, and adhesion formation following fecal peritonitis: An experimental study in the rat. Br J Surg. 1974;61:601–604.
- Watters WB, Buck RC. Scanning electron microscopy of mesothelial regeneration in the rat. Lab Invest. 1972;26:604–609.
- Foley-Comer AJ, Herrick SE, Al-Mishlab T, Prele CM, Laurent GJ, Mutsaers SE. Evidence for incorporation of free-floating mesothelial cells as a mechanism of serosal healing. J Cell Sci. 2002;115:1383–1389.
- Di Paolo N, Sacchi G, Vanni L, Autologous peritoneal mesothelial cell implant in rabbits and peritoneal dialysis patients. Nephron. 1991;57:323–331.
- Hekking LH, Harvey VS, Havenith CE, Mesothelial cell transplantation in models of acute inflammation and chronic peritoneal dialysis. Perit Dial Int. 2003;23:323–330.
- Wagner JC, Johnson NF, Brown DG, Wagner MMF. Histology and ultrastructure of serially transplanted rat mesothelioma. Br J Cancer. 1982;46:294–299.
- Takanobu I, Hiroya M, Sanae K, Improvement of peritoneal membrane used autologous bone marrow mononuclear cells induced recovery of peritoneal function-the relation of number of blood vessels. J Jpn Soc Dial Ther. 2006;39:630 ( abstract).
- Takanobu I, Hiroya M, Kikuchi S, Regeneration of peritoneal mesothelial cell by autologous bone marrow mononuclear cells implantation induces the recover of peritoneal function in a peritoneal dialysis model of rats. J Jpn Soc Dial Ther. 2005;38:654( abstract).
- Sekiguchi Y. Roles of bone marrow-derived cells in development of morphological alterations in the peritoneum. Presented at the 11th Congress of the International Society for Peritoneal Dialysis, Hong Kong, August 25–26, 2006.
- Lucas PA, Warejcka DJ, Zhang LM, Newman WH, Young HE. Effect of rat mesenchymal stem cells on development of abdominal adhesions after surgery. J Surg Res. 1996;62: 229–232.
- Alison MR, Poulsom R, Jeffery R, Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257.
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74.
- Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500.
- Krause DS, Theise ND, Collector MI, Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377.
- Pittenger MF, Mackay AM, Beck SC, Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
- Donna A, Betta PG. Differentiation towards cartilage and bone in a primary tumor of pleura. Further evidence in support of the concept of mesoderma. Histopathology. 1986;10: 101–108.
- Hsiao YC, Chang HH, Tsai CY, Coat color-tagged green mouse with EGFP expressed from the RNA polymerase II promoter. Genesis. 2004;39:122–129.
- Yen TH, Alison MR, Cook HT, The cellular origin and proliferative status of regenerating renal parenchyma after mercuric chloride damage and erythropoietin treatment. Cell Prolif. 2007;40:143–156.
- Yen TH, Chen Y, Fu JF, Proliferation of myofibroblasts in the stroma of renal oncocytoma. Cell Prolif. 2010; 43:287–296.
- Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9:444–450.
- Orlic D, Kajstura J, Chimenti S, Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–715.
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601.
- Lewis WH. Mesenchyme and mesothelium. J Exp Med. 1923;38:257–262.