Abstract
Introduction: Some studies suggest that high body mass index (BMI) confers survival advantage in dialysis patients, but BMI does not differentiate muscle from fat mass, and the survival advantage conferred by its increase seems to be limited to patients with high muscle mass. Thus, discriminating body components when evaluating nutritional status and survival is highly important. This study evaluated the influence of nutritional parameters on survival in patients on chronic dialysis. Subjects and methods: Anthropometry, bioimpedance, biochemistry, and dietary recall were used to investigate the influence of nutritional parameters on survival in 79 prevalent patients on chronic dialysis. Results: Protein intake <1.2 g/kg/day and creatinine <9.7 mg/dL were independent predictors of mortality in all patients. Regarding dialysis method, protein intake <1.2 g/kg/day was predictive of mortality among hemodialysis patients, and percent standard mid-arm muscle circumference <80% was identified as a risk factor among peritoneal dialysis patients. Conclusion: Higher muscle mass, possibly favored by a higher protein intake, conferred survival advantage in dialysis patients.
INTRODUCTION
Some studies suggest that high body mass index (BMI) confers survival advantage in dialysis patients. However, although often used as an indicator of nutritional status, BMI is not a good indicator of body composition because it does not differentiate muscle from fat mass, and the survival advantage conferred by its increase seems to be limited to patients with high muscle mass. Despite a normal or high BMI, some patients also show protein-energy wasting, which includes loss of somatic protein stores, and these patients have been reported to have a high mortality rate.Citation1–4 Moreover, some observational studies have shown a relationship between low protein intake and higher mortality.Citation5,Citation6
Identification of those nutritional indicators related to mortality would allow early intervention and improvement of clinical prognosis. Based on these grounds, discriminating body components, when evaluating nutritional status and survival, is highly important. Thus, the purpose of this study was to assess the influence of nutritional parameters on survival in dialysis patients.
SUBJECTS AND METHODS
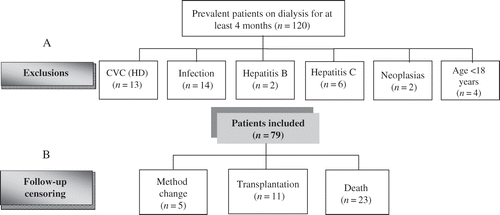
This prospective study, started in 2006, was approved by the institution's Research Ethics Committee. A total of 120 prevalent patients with chronic renal disease on either hemodialysis (HD) or peritoneal dialysis (PD) for at least 4 months at Botucatu Medical School Hospital – UNESP were screened. Exclusion criteria were age less than 18 years, malignancy, central venous catheter use (HD), antibiotic use within 2 months prior to enrollment, positive serologic test for HIV, or hepatitis B or C (A).
Figure 1. Study participants flow. (CVC, central venous catheter; HD, hemodialysis; A, Inclusion and exclusion criteria; B, Follow-up censoring).
Nutritional parameters were obtained at enrollment. Skinfold thickness measurements (triciptal – TSF, biciptal, subscapular, and suprailiac) were taken using a Lange® caliper (Beta Technology®, Santa Cruz, California, USA). BMI was calculated as body weight divided by height squared, whereas percent body fat (%BF) was estimated by the sum of four skinfolds.Citation7,Citation8 Percent standard mid-arm muscle circumference (%MAMC) was calculated using percentile distribution tables adapted by Frisancho.Citation9
Bioimpedance (BIA) measurements were made using a Biodynamics® device (model 450, Biodynamics Corporation®, Seattle, WA, USA). A single frequency current (800 μA and 50 KHz) was applied to assess reactance, phase angle (PA), and %BF.
Laboratory assessment included total lymphocyte count, albumin, creatinine, and C-reactive protein (CRP) for the evaluation of inflammatory status. Blood samples from HD patients were obtained in the predialytic period. Energy and protein intakes were evaluated based on 3-day food recalls using the Nutwin® software (UNIFESP, São Paulo, SP, Brazil), taking into account the energy secondary to dialysate glucose absorption (PD). Values were normalized for patient weight.Citation10
To compare variables between survivors and nonsurvivors, the tests of Wilcoxon or X2 were used, when appropriate. Survival was analyzed using the method of Kaplan–Meier with curves tested by log-rank. Death was the end point event and patients were censored at dialysis modality switch, transplantation, or transfer to another facility. The cutoff points used for nutritional variables were established considering the median values found in the study population or values reported in other studies. A Cox regression model included nutritional variables that showed p < 0.20 in log-rank test and the model was adjusted for age, gender, time on dialysis, CRP, dialysis method, and presence of diabetes mellitus (DM). Mortality hazard ratio (HR) was reported at a 95% confidence interval. Variables were expressed as median (first and third quartiles) or percent as appropriate. Statistical significance was set at p < 0.05. The statistical analysis was conducted using SAS 9.2 (Cary, North Carolina, USA).
RESULTS
Of the 120 patients screened, 79 were actually enrolled in the study. Follow-up length was 38 months, with a median of 33 months (17; 38). Over this period, 29.1% of the patients died. The mortality rate in the first year of follow-up was 10% in HD and 26% in PD patients. Data on follow-up censoring are shown in B.
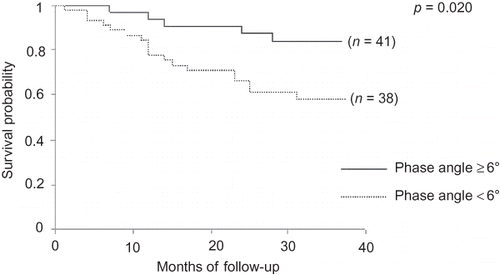
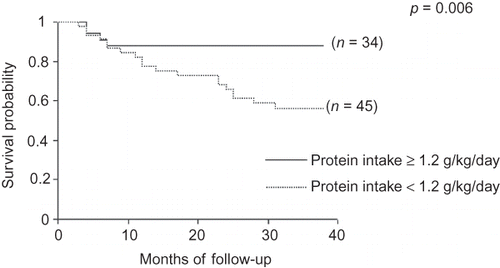
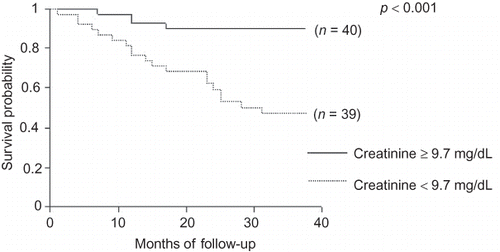
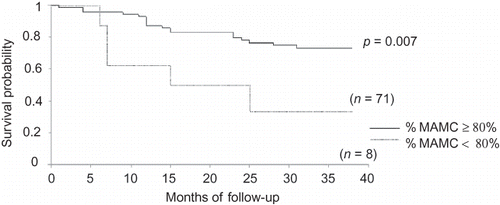
Comparisons between survivors and nonsurvivors are shown in . Nonsurvivors were older, most of them were receiving PD, showed a higher prevalence of DM and lower male prevalence, and had been on dialysis for a longer time. In relation to nutritional parameters, they showed lower PA, reactance, %BF by BIA, creatinine, and energy intake. Survival curves, tested by log-rank, showed that protein intake ≥1.2 g/kg/day, PA ≥ 6°, creatinine ≥ 9.7 mg/dL, and %MAMC ≥ 80% had a significant impact on patient survival ().
Table 1. General characteristics of the study cohort and comparison between survivors and nonsurvivors
Figure 5. Survival curve of dialysis patients according to %MAMC (%MAMC, percent standard mid-arm muscle circumference).
According to multivariate analysis including both HD and PD patients, lower creatinine and protein intake were independent predictors of mortality ().
Table 2. Cox proportional hazards analysis of nutritional factors predicting mortality among dialysis patients (n = 79) adjusted for age, gender, time on dialysis, diabetes, dialysis modality, and C-reactive protein
Regarding dialysis method, protein intake < 1.2 g/kg/day was predictor of mortality among HD patients [HR = 14.52 (95% CI 1.04–208.82), p = 0.047] and %MAMC < 80% was identified as a risk factor among PD patients [HR = 9.75 (95% CI 1.05–96.5), p = 0.050].
DISCUSSION
Protein-energy malnutrition is common in patients receiving dialysis (20–50%) Citation2,Citation11–13 and contributes to the high mortality seen in this population,Citation5,Citation14,Citation15 which in this study was influenced by protein intake and muscle mass markers.
Creatinine is a recognized parameter for nutritional status evaluation, reflecting the muscle mass of dialysis patients and influencing the survival of them, independently of inflammatory state.Citation16 In our study, dialysis patients with creatinine <9.7 mg/dL showed worse survival. This finding is in line with other studiesCitation11,Citation15,Citation16 and is similar to that reported by Lowrie et al.Citation17 according to whom creatinine >10 mg/dL was associated with better outcomes, regardless of the dialytic method used. A positive correlation between creatinine and PA has been previously reportedCitation18,Citation19 and supports the value of PA as a nutritional marker, particularly of muscle mass. Consistent with Mushnick et al.Citation20 worse survival was also observed among our patients with PA < 6° according to univariate analysis.
The recommended protein and energy intakes for stable patients on dialysis are 1.2 g/kg/day and 30–35 kcal/kg/day, respectively.Citation10 In this study, protein intake below recommended levels was observed in 57% of the cases, being predictive of mortality in all study population – when all patients were analyzed together – and among HD patients – when HD and PD patients were investigated separately. This influence is corroborated by others who, in addition to that, found a positive correlation between protein intake and muscle mass estimated by %MAMC.Citation5,Citation21
Honda et al.,Citation22 using subjective global assessment, found malnutrition in patients classified as overweight by BMI, a condition associated with higher mortality, and suggested that the poor prognosis in these patients was mainly due to muscle mass depletion.
In this study, death risk was higher in PD patients with %MAMC < 80%. On the contrary, another national study found an increased risk in HD patients when %MAMC < 90% in analysis adjusted for DM.Citation5 Kakiya et al.Citation23 observed that increased muscle mass and fat mass were both associated with better outcomes. However, in relation to fat mass, a previous study showed that inflamed patients (CRP > 1 mg/dL) had higher BMI and %BF, that may indicate a role for adipose tissue in the pathogenesis of inflammation,Citation24 which is associated with worse survival.Citation25 Moreover, given that central adiposity confers a higher risk of cardiovascular disease than does peripheral subcutaneous fat,Citation4 the importance of body fat distribution has been speculated. In this study, subcutaneous fat alone was assessed, and this may explain why fat was not identified as a predictor of mortality, except when survivors were compared with nonsurvivors.
This study analyzed PD and HD patients together and this might cause interpretation bias, especially regarding laboratory variables. Therefore, for the sake of reliability, analysis was adjusted according to the dialytic method, and the effects of nutritional parameters on PD and HD patients were evaluated separately. It is noteworthy, however, that anthropometric and BIA variables can be affected by the hydration status, and this might have influenced our results.
In conclusion, despite the small sample size, %MAMC, creatinine, and protein intake were independent predictors of mortality, suggesting that higher muscle mass, possibly favored by a higher protein intake, confers survival advantage. These results highlight the importance of dietary counseling and body composition assessment during the follow-up of dialysis patients.
Acknowledgment
The authors thank Mariza Branco for her assistance with the English version.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Beddhu A, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372.
- Chung SH, Linholm B, Lee HB. Is malnutrition an independent predictor of mortality in peritoneal dialysis patients? Nephrol Dial Transplant. 2003;18:2134–2140.
- Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554.
- Mafra D, Guebre-Egziabher F, Fouque D. Body mass index, muscle and fat in chronic kidney disease: Questions about survival. Nephrol Dial Transplant. 2008;23:2461–2466.
- Araújo IC, Kamimura MA, Draibe SA, Nutritional parameters and mortality in incident hemodialysis patients. J Ren Nutr. 2006;16:27–35.
- Dwyer JT, Laurive B, Leung J, Are nutritional status indicators associated with mortality in the hemodialysis (HEMO) study? Kidney Int. 2005;68:1766–1776.
- Siri WE. Body composition from fluid spaces and density: Analysis of methods. 1956. University of California Radiation Laboratory Publication No. 3349.
- Durnin JVGA, Womersley J. Body fat assessment from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged 16 to 72 years. Br J Nutr. 1974;32:77–97.
- Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34: 2540–2545.
- NKF/KDOQI. Clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2000;35:S17.
- Szczech LA, Reddan DN, Klassen PS, Interactions between dialysis-related volume exposures, nutritional surrogates and mortality among ESRD patients. Nephrol Dial Transplant. 2003;18:1585–1591.
- Wang AY, Sea MM, Tang N, Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol. 2004;15:3134–3143.
- Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19:127–135.
- Marcén R, Teruel JL, Cal MA, Gámez C. The impact of malnutrition in morbidity and mortality in stable hemodialysis patients. Nephrol Dial Transplant. 1997;12:2324–2331.
- Terrier N, Jaussent I, Dupuy A, Creatinine index and transthyretin as additive predictors of mortality in hemodialysis patients. Nephrol Dial Transplant. 2008;23:345–353.
- Beddhu S, Cheung AK, Larive B, Inflammation and inverse associations of body mass index and serum creatinine with mortality in hemodialysis patients. J Ren Nutr. 2007;17: 372–380.
- Lowrie EG, Huang WH, Lew NL. Death risk predictors among peritoneal dialysis and hemodialysis patients: A preliminary comparison. Am J Kidney Dis. 1995;26:220–228.
- Vannini FD, Antunes AA, Caramori JCT, Martin LC, Barretti P. Associations between nutritional markers and inflammation in hemodialysis patients. Int Urol Nephrol. 2009;41: 1003–1009.
- Beberashvil I, Sinuani I, Azar A, Nutritional and inflammatory status of hemodialysis patients in relation to their body mass index. J Ren Nutr. 2009;19:238–247.
- Mushnick R, Fein PA, Mittman N, Goel N, Chattopadhyay J, Avram MM. Relationship of bioelectrical impedance parameters to nutrition and survival in peritoneal dialysis patients. Kidney Int. 2003;64:S53–S56.
- Maiorca R, Brunori G, Zubani R, Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant. 1995;10:2295–2305.
- Honda H, Qureshi AR, Axelsson J, Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86: 633–638.
- Kakiya R, Shoji T, Tsujimoto Y, Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int. 2006;70:549–556.
- Antunes AA, Vannini FD, Martin LC, Inflammation and overweight in peritoneal dialysis: Is there an association? Ren Fail. 2009;31:549–554.
- Pecoits-Filho R, Barany P, Linholm B, Heimburger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–1688.