Abstract
Contrast agents are associated with a number of adverse effects, including central nervous system effects. These agents are primarily filtered and excreted by the kidney. Contrast-associated encephalopathy is a rare complication. We report the case of a 55-year-old male on chronic hemodialysis who developed confusion and agitation after receiving ioversol during abdominal angiography. Although hemodialysis was performed his healing took 15 days. Patients with end-stage renal disease may be at an increased risk of adverse effects of contrast agents.
INTRODUCTION
The most important complications of intravascular administration of contrast agents are idiosyncratic reactions, shock, congestive heart failure, cardiac arrhythmias, acute renal failure, and neurotoxic effects.Citation1 Contrast agents are primarily filtered and excreted unchanged by the kidneys. The pharmacokinetics of these agents is significantly altered in the presence of renal failure. As the survival of dialysis patients increases, more radiographic imaging studies are required. We report a case of contrast-induced encephalopathy following abdominal aortography and renal artery angioplasty in a hemodialysis patient.
CASE REPORT
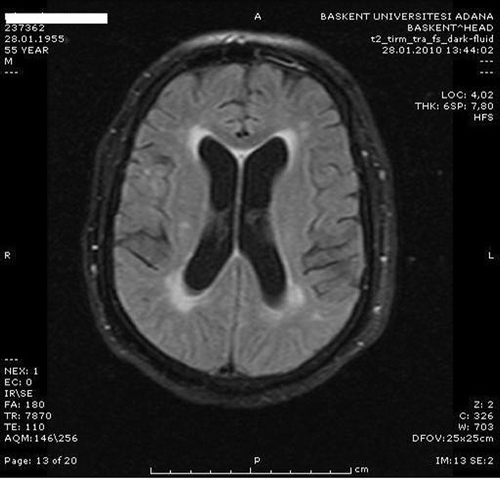
The patient is a 55-year-old male with end-stage renal disease secondary to diabetes mellitus on hemodialysis for 4 years, 3 times a week, through left antecubital arteriovenous fistula. He was admitted to the hospital to work up and to be treated for uncontrolled hypertension. He was taking amlodipine 10 mg/day, losartan 100 mg/day, and carvedilol 50 mg/day, and his dry weight was 107 kg. On physical examination, his blood pressure was 180/100 mmHg and there were no rales and edema. Left ventricular hypertrophy and diastolic dysfunction were detected on echocardiography. Cardiothoracic ratio in chest X-ray was 0.5 and there was no pleural effusion. The dialysis adequacy measured as Kt/V was 1.17. His medical history included no neurological or cerebrovascular disease. Coronary angiography and stenting of left anterior descending (LAD) artery had been performed 5 months ago at another hospital and no complication was observed. During that procedure renal angiography had been done and bilateral renal artery stenosis was detected. Because his blood pressure was under control, intervention was not performed until his blood pressure started to surge. Later he underwent an abdominal digital substraction angiography. Stenosis was 40% in the right renal artery and 60–70% in the left side. There was severe stenosis in the intraparenchymal segment of renal arteries. Angioplasty was performed only for the left side. A total of 100 mL of ioversol was used during the procedure. After 2 h of the procedure, the patient became confused and agitated. His physical examination revealed a disoriented, agitated, and confused patient, blood pressure was 160/90 mm/Hg, pulse 78/min, rhythmic, and neurological examination revealed no focal deficit. His laboratory parameters were as follows: glucose 185 mg/dL, blood urea nitrogen (BUN) 60 mg/dL, creatinine 10 mg/dL, Na 139 mEq/L, potassium 5.9 mEq/L, calcium 8.9 mg/dL, phosphorus 5.9 mg/dL, ALT 11 IU/L, AST 28 IU/L, lactate dehydrogenase (LDH) 185 IU/L, albumin 3.5 g/dL, hemoglobin 9.2 g/dL, and platelets 193 K/mm3. Hemodialysis was performed 3 h after angiography and on the following day, but his clinic evaluation did not change. Cerebral diffusion magnetic resonance imaging (MRI) showed chronic ischemic gliotic lesions in bilateral periventricular white matter and centrum semiovale. There was no acute ischemic lesion, edema, or hematoma (). Cerebrospinal fluid was analyzed after 3 days. Cerebrospinal fluid analysis showed clear fluid with a total cell count of 10, sugar 116 mg/dL, protein 35 mg/dL, and density 1005. Cerebrospinal fluid cultures and gram stains were negative and the pathological evaluation was unremarkable. Electroencephalograph (EEG) showed irregular basal rhythm predominantly consisting of theta-wave activities. As his agitation could not be controlled with haloperidol, we started quetiapine. The dosage of quetiapine was gradually increased to 50 mg/day and agitation was controlled. In 15 days, he was oriented and his agitation improved. Patient's blood pressure control has not changed after the intervention. After all these interventions, he was suggested to have contrast agent encephalopathy.
DISCUSSION
Different types of contrast agents are used in clinical practice. They are classified on the basis of their physical and chemical characteristics (chemical structure, osmolality, iodine content, iodination in solution). Nonionic monomers of low-osmolality contrast media are potentially less chemotoxic than the ionic monomers and ioversol was one of these agents. The osmolality of nonionic monomers is 290–860 mOsm/kg. Dialysability of ioversol was shown previously and the mean dialysance of ioversol was 114–129 mL/min and the elimination rate was 82.5 ± 5.1% 4 h after starting hemodialysis. The half-life of ioversol during hemodialysis was 1.80 ± 0.38 hours.Citation2
Neurological adverse events associated with ioversol are anxiety, blurred vision, confusion, dry mouth, headache, generalized spasm, and vertigo.Citation3 Our patient was confused, agitated, and disoriented. Renografin-76-associated encephalopathy and seizures in a patient with chronic renal insufficiency after cardiac catheterization was previously reported.Citation4 In that patient, computed tomography (CT) of the brain, immediately post cardiac catheterization had demonstrated hyperdensity in the left frontal gyrus without surrounding edema or mass effect and epileptiform activity on electroencephalograph. Seizure was controlled with hemodialysis. CT findings were resolved in 48 hours.Citation4 On the contrary, MRI showed neither edema nor acute ischemic lesions and hemodialysis did not change neurological findings in our patients. In another report of three cases, encephalopathy was observed after carotid or cerebral arteriography. They were not dialysis patients and all were elderly men being evaluated for cerebrovascular disease and they demonstrated global cerebral dysfunction as a complication of their arteriogram. CT scan disclosed no abnormalities.Citation5
Increased levels of the contrast agents in the blood may be a risk factor for neurotoxicity. Neurotoxic effects of the contrast agents may be associated with their hyperosmolality resulting in hemodynamic and blood–brain barrier alteration.Citation4 Once a contrast agent penetrates the brain, it can cause cortical dysfunction by increasing neuronal excitability.Citation6 Although ioversol is dialyzable and one of the hydrophilic nonionic low-osmolality contrast agents, presence of chronic renal failure and diabetes could have effected the susceptibility of neurons and it has caused encephalopathy. Furthermore, the exposure of contrast agent 5 months ago could be one of the susceptibility factors. Because of the presence of diabetes and renal failure, it took 15 days for his improvement.
In conclusion, the encephalopathy seen in our patient may have been caused by direct toxic effect of ioversol on the brain. Contrast agents should be thought as a cause of neurological disturbances in patients with renal failure. Hemodialysis should be performed right after exposure of the contrast agent to prevent these adverse effects, and risks and benefits of the intervention always should be weighed.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Junck L, Marshall WH. Neurotoxicity of radiological contrast agents. Ann Neurol. 1983;13(5):469–484.
- Ueda J, Furukawa T, Takahashi S, Sakaguchi K. Elimination of ioversol by hemodialysis. Acta Radiol. 1996;37(5):826–829.
- Floriani I, Ciceri M, Torri V, Tinazzi A, Jahn H, Noseda A. Clinical profile of ioversol. A metaanalysis of 57 randomized, double-blind clinical trials. Invest Radiol. 1996;31(8):479–491.
- Muruve DA, Steinman TI. Contrast-induced encephalopathy and seizures in a patient with chronic renal insufficiency. Clin Nephrol. 1996;45(6):406–409.
- Haley EC Jr. Encephalopathy following arteriography: A possible toxic effect of contrast agents. Ann Neurol. 1984;15(1):100–102.
- Torvik A, Walday P. Neurotoxicity of water-soluble contrast media. Acta Radiol Suppl. 1995;399:221–229.