Abstract
Background: Even though IgA nephropathy (IgAN) is not the most common primary glomerulonephritis (GN) in India, the outcome of patients with IgAN in India is poor when compared with other parts of the world, which is a burden in itself. Methods: Basic and clinical research work in India on primary IgAN was systematically reviewed. Comparisons between data from India and those from other countries were made. Results: IgAN constitutes between 7% and 16% of most biopsy samples from India, bearing in mind these figures may represent only the tip of the iceberg. Nephrotic syndrome and renal failure seem to be common presenting features. The renal survival rates appear to be dismally low. DD genotype of angiotensin-1 converting enzyme (ACE) gene may predispose the individual to IgAN in Indian population. As might be expected, IgAN can recur posttransplant though the posttransplant course is indolent. There are no data on the treatment aspects of IgAN. Conclusion: Low incidence but marked severity characterizes IgAN in India. It is apparent that IgAN seems to have a poor outcome in India. What we are unsure of is the reason behind it. In-depth basic studies and multicenter clinical trials are needed to address this bizarre pattern.
INTRODUCTION
IgA nephropathy (IgAN) is the most common cause of primary (idiopathic) glomerulonephritis (GN) in the developed world.Citation1 Although this disorder was initially thought to follow a benign course, it is now recognized that slow progression to end-stage renal disease (ESRD) occurs in up to 50% of affected patients.Citation2 The remaining patients enter a sustained clinical remission or have persistent low-grade hematuria or proteinuria. However, the prognosis is quite variable and the outcome difficult to predict with accuracy in individual patients.
The classic presentation of IgAN is that of gross hematuria, often recurrent, following shortly after an upper respiratory infection.Citation3 However, the majority of patients are diagnosed following an evaluation for asymptomatic microscopic hematuria and/or mild proteinuria. Less common presentations are nephrotic range proteinuria with or without nephrotic syndrome and rapidly progressive GN and, rarely, malignant hypertension. The diagnosis is usually suspected based on the clinical history and laboratory data but can only be confirmed with a kidney biopsy, at the present time. There is now a reasonably complete understanding of the pathogenesis and mediation of this disease, but its etiology remains obscure and mysterious. New data on epidemiology and genetic predisposition continue to emerge that will undoubtedly have clinical significance.
In India, however, primary IgAN continues to be a mystery. Initial studies have speculated that the relatively rapid rate of progression of IgAN in India is suggestive toward a “malignant” nature of the disease in this country. This review systematically examines the disease from an Indian perspective.
PREVALENCE
The world-wide frequency of IgAN varies widely from 2% to 52% of all renal biopsies.Citation4 There is a definite geographical variation. Available evidence from India suggests a prevalence of ∼7–16%.Citation4–8 We had previously published the histopathological data of 5415 adequate renal biopsies from a tertiary hospital in southern India.Citation4 In this study, IgAN constituted 8.6% of the total biopsies. Similar observations were made from other parts of the country with prevalence of 7.24% from New Delhi in North India on a cohort of 1146 patientsCitation5 and 10.37% from the North Indian Union Territory of Chandigarh.Citation6 In a study on 1592 renal biopsies from the southern state of KeralaCitation7 revealed a prevalence of 14.26%, whereas in that from western India (n = 4132) it was 16.2%Citation8 ().
Table 1. IgA nephropathy studies from India
Frequency reported from North America, the United Kingdom, and Northwestern Europe varied from 5% to 10%.Citation9–13 In Europe, the highest was in Italy (35.9%),Citation14 followed by France (30.1%).Citation15 Among Asian countries, it ranged from 25% to 52%, with Singapore (52%)Citation16 and Japan (47.2%)Citation17 among countries with the highest incidence.
In a study from South Africa, the authors reported a very low prevalence in the black population (0.7%) as compared with Indian residents (13.3%).Citation18 This supports the conclusion from Yoshino et al.’s study that race and ethnicity are important factors affecting the distribution of IgAN.Citation19
Biopsy policy is another important factor affecting the frequency of IgAN as has been pointed out in a study from the United Kingdom in which the frequency varied from 7.1% (1972–1978) to 21.1% (1979–1986).Citation20 The biopsy policy varied depending on the nephrologists and the availability of immunofluorescence facility. Although these disparities may reflect differences in public health awareness or the willingness of nephrologists to perform diagnostic biopsies, certain populations seem to have a genetic predisposition to the development of IgAN.Citation21
CLINICAL PRESENTATION
Patients with IgAN typically present in one of three ways; the relative frequency depends partly on screening practices (which will lead to increased discovery of asymptomatic cases) and the particular population being evaluated.Citation3 Approximately 40–50% present with one or recurrent episodes of gross hematuria, usually following an upper respiratory infection. This has sometimes been called “synpharyngitic hematuria.” Another 30–40% have microscopic hematuria and usually mild proteinuria and are incidentally detected on a routine examination.Citation22,Citation23 Less than 10% present with either nephrotic syndrome or acute rapidly progressive GN picture characterized by edema, hypertension, and renal insufficiency as well as hematuria.
Indian adults with IgAN presenting with nephrotic syndrome or renal failure are not uncommon. It is usually presumed that these patients have a long-standing disease that was not detected earlier because the patient did not undergo routine urinalysis. Thus, the clinical presentation of IgAN in Indian adults differed from the existing norm. In a retrospective study involving 478 patients of biopsy-proven IgAN, the mean age at presentation was 32 (±11) months, with a predominant male (65%) cohort. Nephrotic syndrome (55%), renal failure (serum creatinine > 1.4 mg/dL in 60%), hypertension (58%), and acute nephritis (16%) were the commonest modes of presentation.Citation24 Clinical features that were unusually severe in a high proportion were also found in other studies from India (). Restrictive biopsy policies with consequent “selection” of the population whose clinical course is closely monitored might explain this variation in the modes of presentation.
PATHOLOGICAL CHARACTERISTICS
In its “classic” presentation, IgAN is characterized by a mesangiopathic process with expansion of the mesangial matrix, immunofluorescent staining for IgA, and proliferation of mesangial cells. The histological presentation of IgAN is not limited to these findings but like other immune complex GN can exhibit more aggressive histology, including crescents, karyorrhexis, and endocapillary proliferation. Up until the recent OxfordCitation25 classification of IgAN, histological classification was based on the Haas's classification.Citation26 The same has been used in the two studies concentrating on histology. The Vellore groupCitation24 reported a higher frequency of class III (focal proliferative GN, 50%), followed by class II (focal and segmental glomerulosclerosis without active cellular proliferation, 25%), class IV (diffuse proliferative of GN, 15%), and class V (≥40% globally sclerotic glomeruli and/or ≥40% tubular atrophy or loss, 10%).
Studies from other centersCitation7 revealed that class II (40.5%) had the highest frequency followed by class V (28.6%). Extensive glomerular crescents and sclerosis were prominent in the study by Bhuyan et al.,Citation5 who also noticed moderate arteriolitis, and arteriolosclerosis and marked tubulointerstitial nephropathy as notable features.
PROGRESSION AND OUTCOME
Patients with IgAN and little or no proteinuria (<500 mg/day) have a low risk of progression in the short term. However, renal insufficiency and proteinuria develop in a substantial proportion of patients over the long term, and such patients may progress to ESRD.Citation1,Citation2 The possible role of persistent microscopic hematuria in predicting an adverse outcome in this group of patients is debated. The impact of different biopsy criteria was shown in a retrospective study that evaluated geographical differences in the clinical course of 711 patients with a biopsy diagnosis of IgAN.Citation2 Renal survival at 10 years was 96%, 87%, 64%, and 62% in Finland, Australia, Scotland, and Canada, respectively. Better outcomes in Finland and Australia were largely, although not completely, attributable to the diagnosis of milder cases (e.g., less proteinuria, higher creatinine clearance, lower blood pressure). This raises the possibility of lead-time bias influencing the prognosis versus truly a better prognosis in some geographical areas compared with others.Citation2,Citation27
Hypertension, proteinuria, and renal insufficiency have been traditionally described as progression factors for IgAN.Citation1 This more or less holds good for IgAN in India too, though there are only two studies addressing prognosis and outcome. Comparisons between studies from different countries are generally difficult because of differences in patient populations, stages of the disease, and follow-up periods and it is known that some factors related to progression are associated with decreased renal function per se. To avoid bias, factors associated with progression were analyzed separately for groups defined on the basis of level of renal function at diagnosis. In this study,Citation24 only the existence and the degree of hypertension and impaired creatinine clearance at the time of diagnosis were associated with progression at all stages of the disease on the biopsy that were defined and studied. Higher levels of proteinuria, serum triglyceride, and serum uric acid were statistically associated with progression in patients with renal insufficiency at presentation.
Interstitial fibrosis, sclerosed glomeruli, tubular changes, and the intensity of IgM deposits were significantly related to progression in those with renal insufficiency at presentation but not in those with normal renal function. The impact of histological alterations as prognostic indicators in the early stage of the disease is minimal. On multivariate analysis, factors independently predictive of outcome were hypertension, nephrotic range proteinuria, and greater than 50% of sclerosed glomeruli on biopsy. This, however, was applicable only to patients with renal failure. In patients with normal renal function, hypertension alone was an independent predictive factor for poor outcome.
The cumulative probability of renal survival (i.e., not progressing to ESRD) at 1, 5, and 10 years was from the onset of symptoms 0.91, 0.64, and 0.35, respectively. On multivariate analysis, four factors (hypertension, nephrotic range proteinuria, degree of sclerosed glomeruli, and interstitial fibrosis) were found to be significantly associated with progression to ESRD. Patients without identified risk factors (hypertension, nephrotic range proteinuria, and >50% sclerosed glomeruli) had a mean renal survival time of 132 months, whereas the mean survival time in those with one, two, or all three risk factors was 105, 47, and 28 months, respectively ().
Figure 1. Cumulative probability of renal survival and presence of risk factors. Risk factors include hypertension, nephrotic proteinuria, interstitial fibrosis, and sclerosed glomeruli (adapted from Chacko et al.Citation24 with permission).
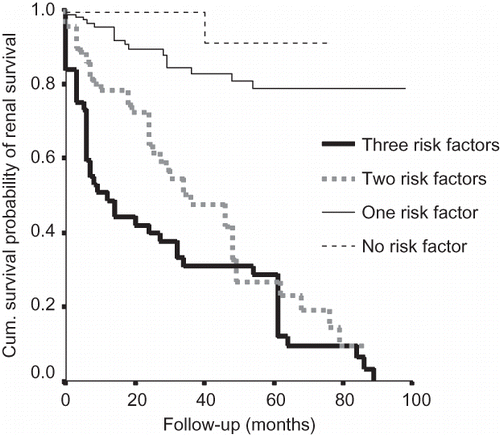
Seventeen percent of patients progressed to ESRD and a further 29% had more than a 20% permanent decline in renal function at last follow-up. Renal survival at 10 years was a dismal 35% when compared with nearly 90% in studies from Singapore,Citation16 Australia,Citation28 and FranceCitation29 (). The variability at presentation in both duration (lead-time bias) and severity of disease (higher level of mean serum creatinine (2.3 mg/dL) at the time of presentation in this study) may explain this discordance to a great extent. However, the possibility of other factors (genetic and environmental) contributing to this poor prognosis warrants consideration. Interestingly, a US studyCitation26 with levels of mean serum creatinine comparable with this study had a relatively better 10-year survival rate (57% and 33%). Obviously, the concept of lead-time bias alone cannot explain the variability in the renal survival rate around the world. Race, genes, environment, and treatment modalities also play a role. Schena et al.Citation30 have demonstrated increased risk of ESRD in familial IgA when compared with those with sporadic disease.
Table 2. Outcome of IgA in India compared with rest of the world
A rather disturbing trend observed in this study was the progressive nature of renal dysfunction and poor renal survival, seen in patients diagnosed from the year 2000 onward, as opposed to those before the year 2000 despite stratification for differences in mean serum creatinine between cohorts. Whether the present genetic and environmental milieu and lifestyle changes are responsible for this is highly speculative.
In the only other studyCitation31 from India, addressing outcome, age >25 years, glomerular histology of Haas-V, and interstitial fibrosis were significant factors for progression. Forty-eight percent patients developed ESRD during follow-up. Serum creatinine >5 mg/dL, Haas-V, crescents, and interstitial fibrosis were associated with disease progression in the multivariate analysis. Five-year renal survival was 38.5%. Among those with no risk factor, 95% had not reached ESRD by 42 months. The median renal-free survival with one, two, and three or four risk factors was 65, 16, and 4 months, respectively.
In a search for early markers of tubulointerstitial changes to prognosticate renal functions, Minz et al.Citation32 reported that immunohistochemistry for α-smooth muscle actin and type IV collagen provides an easier method of grading interstitial fibrosis (in comparison with existing cumbersome histological grading methods) into good and bad prognostic groups.
MODEL FOR PREDICTING OUTCOME
There is a need to determine the prognosis more precisely in individual patients rather than in groups of patients. Several prediction formulas have been devised, most of them based on relatively simple clinical factors present at discovery or short-term follow-up.Citation33
A similar model was attempted for IgAN in Indian patients. Cox's proportional hazard model was used as it estimates the quantitative effect of each significant risk factor and allows the calculation of a relative risk. Nevertheless, this model assumes that the effect of each risk factor remains constant with time, and we do not know whether it is true for IgAN. From the analysis of our cohortCitation24 of patients, Cox's regression model allowed us to find four significant risk factors for the development of ESRD and gave for each of them their relative weight (β-coefficient). In addition to this, we can estimate the probability of renal survival with the following equation:
The following are the β-coefficients of the four significant risk factors:
X1 = Hypertension (present – 1; absent – 0); β1 = 1.139
X2 = Proteinuria (>3 g/day is 1; 0–3 g/day is 0); β2 = 0.67
X3 = Global sclerosis (>50% is 1; <50% is 0); β3 = 0.59
X4 = Interstitial fibrosis (moderate is 1; mild is 0); β4 = 0.93
Baseline hazard h0(t): 0.04 at 1 year
For example,
Consider a patient with IgAN with hypertension, nephrotic range proteinuria, moderate to severe interstitial fibrosis, and more than 50% of sclerosed glomeruli on biopsy.
Substituting X and â in the Cox's equation:
The probability of a patient with IgAN, having all four risk factors, not progressing to ESRD at 1 year is 0.31. That is, there is a 69% probability that he will develop ESRD at 1 year. There is a need to characterize and validate these risk factors in detail to be able to design and carry out appropriately powered, randomized, controlled clinical trials of treatment.
GENE POLYMORPHISMS: SUSCEPTIBILITY AND PROGRESSION
A number of genetic associations have been suggested to be prognostically important in patients with IgAN, but the data are often conflicting and may be confounded by the population studied. In some studies, progressive disease appeared more likely in patients with the DD genotype of the angiotensin-1 converting enzyme (ACE) gene, which is associated with higher plasma ACE levels, than in those with the ID or II genotype.Citation34–36 However, others have reported no correlation between genotype and outcome.Citation37,Citation38 In a case–control study from India,Citation39 22 patients with IgAN were divided into progressors (n = 13) and non-progressors (n = 9) based on whether there was at least a 20% decline in renal function at last follow-up. DD genotype of ACE gene was shown to predispose the individual to IgAN in Indian population besides having an impact on disease progression and may explain the poor prognosis of IgAN in India. Compared with controls, the IgAN group as a whole showed a different genotype distribution with a trend for a higher frequency of DD genotype (II: 53%, ID: 37%, and DD: 10% vs. II: 27%, ID: 50%, and DD: 23%; p = 0.25), predicting that individuals with DD genotype may be at an increased risk for IgAN. The ACE I/D genotype distribution in IgAN-progressor group was significantly different when compared with IgAN-non-progressor group (II: 17%, ID: 73%, and DD: 80% vs. II: 83%, ID: 27%, and DD: 20%; p = 0.05), indicating that ACE genotyping did prognosticate in IgAN in India.
RENAL TRANSPLANTATION AND IgAN
Transplantation is the treatment of choice for individuals with progressive renal failure because of IgAN Recurrent IgA deposition, as determined on biopsy, may result in a wide spectrum of manifestations, ranging from an incidentally noted histological finding to mesangioproliferative GN associated with hematuria, proteinuria, and progressive renal dysfunction.Citation40
The outcomes of 56 transplant patients (58 grafts) with biopsy-proven IgAN and of 116 patients without IgAN or diabetic nephropathy, transplanted during the same period, were analyzed in a case–control study from India.Citation41 Five-year graft survival for IgA patients was not significantly different from that in the reference group (90% and 79%, p = 0.6). During a mean follow-up of 42 months (range 1–144), 28 event graft biopsies were required in 20 grafts of IgAN. Histological recurrence was diagnosed in 5 of the 20 available biopsies (25%) after a mean duration of 28 months. Recurrence did not correlate with donor status, HLA B35 and A2, recipient age, gender, or immunosuppression. Renal transplantation is an appropriate treatment modality for IgAN patients with ESRD in India, despite the potential for recurrent disease. The posttransplant course is an indolent one when compared with the malignant pretransplant phase.
CONCLUSIONS
Low incidence but marked severity characterizes IgAN in India. It is apparent that IgAN seems to have a poor outcome in India. What we are unsure of is the reason behind it. Genetic polymorphisms alone cannot explain the differences in outcome. There is an urgent need to prognosticate patients with IgAN and advocate therapy in whatever form. In-depth basic studies and multicenter clinical trials are needed to address this bizarre pattern. Efforts toward understanding the natural history and treatment of IgAN in India is needed to reduce the burden of chronic kidney disease and the number of patients who will require renal replacement therapy.
REVIEW CRITERIA
Material for this review was obtained by searching PubMed and MEDLINE databases using the following search items: “IgA,” “Immunoglobulin A nephropathy,” “India,” “progression,” “outcome,” “transplantation,” and “gene polymorphisms.” No limitations were placed on language or year of publication.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- D'Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004;24:179.
- Geddes CC, Rauta V, Gronhagen-Riska C, A tricontinental view of IgA nephropathy. Nephrol Dial Transplant. 2003;18:1541.
- Galla JH. IgA nephropathy. Kidney Int. 1995;47:377.
- Narasimhan B, Chacko B, John GT, Korula A, Kirubakaran MG, Jacob CK. Characterization of kidney lesions in Indian adults: Towards a renal biopsy registry. J Nephrol. 2006 Mar–Apr; 19(2):205–210.
- Bhuyan UN, Dash SC, Srivasthava RN, Tiwari SC, Malhotra KK. IgA associated glomerulonephritis. J Assoc Physicians India. 1992;40:310–313.
- Sehgal S, Datta BN, Sakhuja V, Chugh KS. Primary IgA nephropathy: A preliminary report. Indian J Pathol Microbiol. 1995;38:233–237.
- Chandrika BK. Non-neoplastic renal diseases in Kerala, India–analysis of 1592 cases, a two year retrospective study. Indian J Pathol Microbiol. 2007 Apr;50(2):300–302.
- Vanikar AV, Kanodia KV, Patel RD, Trivedi HL. Primary IgA nephropathy in Western India. Indian J Nephrol. 2005; 15:227–231.
- Hood SA, Velosa JA, Holley KE, Donadio JV Jr. IgA-IgG nephropathy; predictive indices of progressive disease. Clin Nephrol. 1981;16:55–62.
- McCoy RC, Abramowsky CR, Tisher CC. IgA nephropathy. Am J Pathol. 1974;76:123–144.
- Katz A, Underdown BJ, Minta JD. Glomerulonephritis with mesangial deposit of IgA unassociated with systemic disease. Can Med Assoc J. 1976;114:209–215.
- Sissons JG, Woodrow DF, Curtis JR, Isolated glomerulonephritis with mesangial IgA deposits. Br Med J. 1975; 3:611–614.
- Ballardie FW, O'Donoghue DJ, Feehally J. Increasing frequency of adult IgA nephropathy in the UK? Lancet. 1987; 2:1205.
- Schena FP. Survey of the Italian Registry of Renal Biopsies: Frequency of the renal diseases for 7 consecutive years: The Italian Group of Renal Immunopathology. Nephrol Dial Transplant. 1997;12:418–426.
- Simon P, Ang KS, Bavay P, Immunoglobulin A glomerulonephritis: Epidemiology in a population of 250 000 inhabitants. Presse Med. 1984;13:257–260.
- Woo KT, Edmonson RP, Wu AY, Chiang GS, Pwee HS, Lim CH. The natural history of IgA nephritis in Singapore. Clin Nephrol. 1986;25:15–21.
- Koyama A, Igarashi M, Kobayashi M. Natural history and risk factors of immunoglobulin A nephropathy in Japan. Am J Kidney Dis. 1997;29:526–532.
- Seedat YK, Nathoo BC, Parag KB, Naiker IP, Ig RR. A nephropathy in Blacks and Indians of Natal. Nephron. 1988;50:37–41.
- Yoshino NH, Eloisa FF, Glenn MC, Jean LO. Race/ethnicity and disease severity of IgA nephropathy. BMC Nephrol. 2004;5:10.
- Ballardie FW, O'Donoghue DJ, Feehally J. Increasing frequency of adult IgA nephropathy in UK? Lancet. 1987;2:1205.
- D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 2001;64:709–727.
- Hall CL, Bradley R, Kerr A, Clinical value of renal biopsy in patients with asymptomatic microscopic hematuria with and without low-grade proteinuria. Clin Nephrol. 2004; 62:267.
- Topham PS, Harper SJ, Furness PN, Glomerular disease as a cause of isolated microscopic hematuria. Q J Med. 1994;87:329.
- Chacko B, John GT, Neelakantan N, Presentation, prognosis and outcome of IgA nephropathy in Indian adults. Nephrology (Carlton). 2005 Oct;10(5):496–503.
- Roberts IS, Cook HT, Troyanov S, The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546.
- Haas M. Histologic subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis. 1997; 29:829.
- Coppo R, D'Amico G. Factors predicting progression of IgA nephropathies. J Nephrol. 2005;18:503.
- Nicholls KM, Fairley KF, Dowling JP, Kincaid-Smith P. The clinical course of mesangial IgA associated nephropathy in adults. Q J Med. 1984;53:227–250.
- Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18:12–19.
- Schena FP, Cerullo G, Rossini M, Increased risk of end‐stage renal disease in familial IgA nephropathy. J Am Soc Nephrol. 2002 Feb;13(2):453–460.
- Muthukumar T, Fernando ME, Jayakumar M. Prognostic factors in immunoglobulin-A nephropathy. J Assoc Physicians India. 2002 Nov;50:1354–1359.
- Minz RW, Bakshi A, Chhabra S, Joshi K, Sakhuja V. Role of myofibroblasts and collagen type IV in patients of IgA nephropathy as markers of renal dysfunction. Indian J Nephrol. 2010 Jan;20(1):34–39.
- Bartosik LP, Lajole G, Sugar L, Cattran D. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;58: 551–553.
- Harden PN, Geddes C, Rowe PA Polymorphisms in angiotensin-converting-enzyme gene and progression of IgA nephropathy. Lancet. 1995;345:1540.
- Yoshida H, Mitarai T, Kawamura T, Role of the deletion polymorphism of angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J Clin Invest. 1995;96:2162.
- Hunley TE, Julian BA, Phillips JA III, Angiotensin converting enzyme gene polymorphism: Potential silencer motif and impact on progression in IgA nephropathy. Kidney Int. 1996;49:571.
- Suzuki S, Suzuki Y, Kobayashi Y, Insertion/deletion polymorphism in ACE gene is not associated with renal progression in Japanese patients with IgA nephropathy. Am J Kidney Dis. 2000;35:896.
- Schena FP, D'Altri C, Cerullo G, ACE gene polymorphism and IgA nephropathy: An ethnically homogeneous study and a meta-analysis. Kidney Int. 2001;60:732.
- Chacko B, Raghuraman S, Neelakantan N, John GT, Jacob CK. Impact of ACE gene polymorphisms on the progression of IgA nephropathy in India. Ren Fail. 2007;29(5):647–648.
- Kowalewska J, Yuan S, Sustento-Reodica N, IgA nephropathy with crescents in kidney transplant recipients. Am J Kidney Dis. 2005;45:167.
- Chacko B, George JT, Neelakantan N, Korula A, Chakko JK. Outcomes of renal transplantation in patients with immunoglobulin A nephropathy in India. J Postgrad Med. 2007 Apr–Jun; 53(2):92–95.