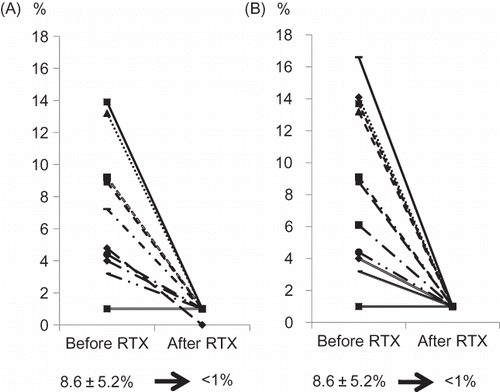
Abstract
High baseline anti-ABO antibody titer is still an important obstacle for successful ABO-incompatible kidney transplantation (ABO IKT). This study aims to investigate the clinical outcome of ABO IKT in patients with a high baseline titer in comparison with patients with a low baseline titer. Fourteen patients who received ABO IKT at our center were classified as the high-titer group (≥1:256, n = 8) or the low-titer group (≤1:128, n = 6). We used a protocol composed of rituximab, plasmapheresis, and intravenous immunoglobulin (RTX/PP/IVIG). We compared the intensity of preparation, complications, and clinical outcome between the two groups. The high-titer group required more sessions of pretransplant (10.5 ± 3.5 vs. 6.0 ± 1.3 times, p = 0.01) and posttransplant (1.6 ± 1.8 vs. 0 ± 0 times) PP/IVIG than the low-titer group did. All patients from both groups showed immediate recovery of graft function. The antibody titer and allograft function in the high-titer group were stable and did not differ significantly from those of the low-titer group up to 1 year after kidney transplantation. There was no antibody-mediated rejection in either group during follow-up, but three cases of acute cellular rejection developed in the high-titer group. The high-titer group showed two cases of opportunistic viral infection (herpes gingivitis and cytomegalovirus viremia) and one case of graft loss due to postoperative bleeding. ABO IKT can be safely performed even in patients with a high baseline anti-ABO antibody titer, but the risk for infection and bleeding should be considered before transplantation.
INTRODUCTION
Organ shortage is a critical issue in Korea as well as in other countries. In Korea, the number of end-stage renal disease patients on the wait list was 8488 in 2009, but only 1295 patients received transplantation during this period.Citation1 Several strategies were developed to overcome organ shortage. ABO-incompatible kidney transplantation (ABO IKT) is a feasible option to increase donor supply. An exchange donor program has been presented as a solution, but it has the limitation that it cannot be applied to blood type O recipients.Citation2
The first ABO IKT was reported in 1955 but was unsuccessful because of the absence of an effective preparation protocol for antibody removal.Citation3 In the 1980s, Alexandre used a protocol to remove anti-ABO antibodies for the first time, but the outcome was still inferior to that of ABO-compatible kidney transplantation (KT).Citation4,Citation5 Since 2000, with the advancement of immunosuppression and plasmapheresis, the outcome of ABO IKT has improved to a great extent and is comparable to that of ABO-compatible KT nowadays.
Despite this improvement, high baseline anti-ABO antibody titer is still an important obstacle for successful KT. Therefore, some centers recommended kidney-paired donation before considering desensitization, especially for patients with a baseline anti-ABO antibody titer equal to or higher than 1:256.Citation6 This means that patients with a high baseline titer have relatively low chances to receive KT. In this report, we present our experiences with ABO IKT in patients with a high baseline anti-ABO antibody titer. In addition, we compared their clinical course to that of patients with a low titer at baseline in our center.
SUBJECTS AND METHODS
Patients and Methods
From April 2009 to June 2010, 14 cases of ABO IKT have been performed in Seoul St. Mary's Hospital. We retrospectively reviewed the medical records of those patients. At baseline, patients with a baseline anti-ABO antibody titer of equal to or higher than 1:256 were regarded as the high-titer group, and patients with a baseline titer of equal to or less than 1:126 were classified as the low-titer group. We collected the baseline characteristics including age, gender, the relationship between recipients and donors, and the blood type of donor and recipient. Immunological tests such as a cross-match test and a panel-reactive antibody (PRA) screening were performed before KT. When the PRA test was positive, we checked the donor-specific HLA antibody using the Luminex method. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital.
Preparation Protocol
Rituximab, plasmapheresis, and intravenous immunoglobulin
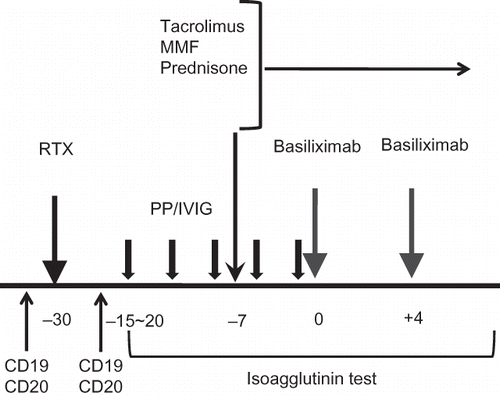
Our center's protocol for ABO IKT is as follows (): We used an anti-CD20 monoclonal antibody (RituximabTM, Genentech Inc., San Francisco, CA, USA; 375 mg/m2) at 30 days before transplantation. We planned the number of pretransplant PP/IVIG based on the widely accepted guideline.Citation7 PP was performed using a COBE Spectra (Gambro BCT, Lakewood, CO, USA) and 1 plasma volume was removed per treatment and replaced with a 5% albumin solution. From 1 day before transplantation to the posttransplant period, we used replacement fluid composed of half of a 5% albumin solution and half of fresh frozen plasma. IVIG (200 mg/kg) was infused 1 h after each PP session. When the antibody titer did not reach the target titer (1:32), additional PP/IVIG was performed. Posttransplant PP/IVIG was performed only when the titer increased to over 1:32.
Figure 1. Our center's protocol for ABO-incompatible kidney transplantation (ABO IKT). We used RTX at 30 days before transplantation and started plasmapheresis and intravenous immunoglobulin (PP/IVIG) at 15–20 days before KT. We started immunosuppressants composed of tacrolimus, prednisone, and mycophenolate mofetil at 7 days before KT. CD19- and CD20-cell counts were measured before and at 14 days after RTX infusion. Isoagglutinin test for anti-ABO antibody was checked daily from the start of PP/IVIG to second week from KT.
Note: RTX, rituximab; MMF, mycophenolate mofetil; PP/IVIG, plasmapheresis/intravenous immunoglobulin.
Immunosuppression and induction therapy
We initiated immunosuppressant therapy 7 days before transplantation. The immunosuppression regimen was composed of tacrolimus (0.1 mg/kg/day), mycophenolate mofetil (2 g/day), and steroid (30 mg). The target trough level of tacrolimus was 10–15 mg/mL. Induction therapy using basiliximab (20 mg) was applied on the day of surgery and on postoperative day (POD) 4.
Monitoring of the anti-ABO antibody titerand B-cell counts
Anti-ABO antibody (IgG/IgM) was checked daily from the initiation of PP/IVIG until the second week after transplantation. At first, the target titer at transplantation and during the first 2 weeks after transplantation was 1:16. Nonetheless, we subsequently increased the target titer to 1:32. Peripheral CD19- and CD20-cell counts were measured by flow cytometry before and at 14 days after RTX infusion.
Monitoring and prophylaxis of infection
For the prophylaxis of opportunistic infections, all patients received fluconazole and bactrim from POD 7 until 6 months after KT. Cytomegalovirus viremia (CMV) was monitored weekly by CMV Real Time PCR (RT-PCR) after KT. When the viral count rose to above 500 copies/mL, we used ganciclovir as a preemptive therapy.
Modification of the protocol
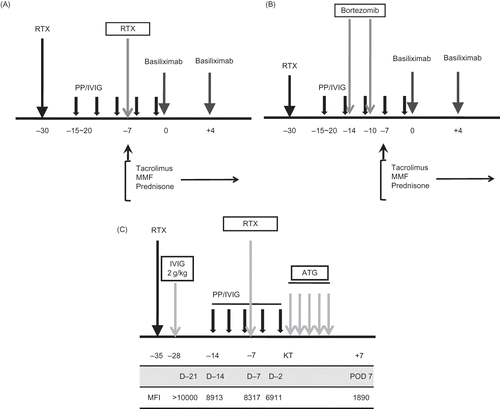
From the third case (high-titer 2) on, we divided the RTX dose into 375 mg/m2 and 100 mg/m2. In patients with a baseline titer higher than 1:512, who had additional risk factors such as sensitization to the HLA antibody (PRA >50%, positive cross-match), or in patients with a previous transplant history, we continued to use 375 mg/m2. In patients with no additional risk, we reduced the RTX dose to 100 mg/m2. We applied a modified protocol in three patients with an extremely high baseline titer (1:1024). In the first (high-titer 1) and the ninth (high-titer 4) patient, we administered RTX (375 mg/m2) twice, at 30 days and 7 days before KT (A). In the 14th patient (high-titer 8), we administered RTX (375 mg/m2) only once at 30 days before KT, and bortezomib (1.3 g/m2) twice at 14 and 10 days before KT (B). In the 10th patient (low-titer 6), in whom strong donor-specific antibody was detected, a modified protocol that was composed of a high dose of IVIG (2 g/kg), RTX administration twice (375 mg/m2), and induction using an anti-thymocyte globulin (Thymoglobulin; Sangstadt, Menlo Park, CA, USA) was used (C).
Figure 2. (A) Modified protocol for patients with extremely high baseline titer (1:1024). In addition to routine protocol, we used additional dose of RTX (375 mg/m2) at 7 days before KT in high-titer 1 and 4. (B) Another modified protocol for patients with extremely high baseline titer (1:1024). In addition to the routine protocol, we used two times of bortezomib at 14 days and 10 days before KT in high-titer 8. (C) Modified protocol for highly sensitized and ABO-incompatible transplantation (ABO IKT) in highly sensitized patients (low-titer 6). In addition to routine protocol for ABO IKT in our center, high-dose intravenous immunoglobulin (2 g/kg) at 28 days before KT and additional dose of RTX at 7 days before KT were used. For the monitoring of donor-specific HLA antibody, Luminex method was used at every week from 3 weeks before KT until 1 week after KT. MFI of the donor-specific anti-HLA antibody gradually decreased from >10,000 to less than 2000 by preparation protocol.
Note: RTX, rituximab; PP/IVIG, plasmapheresis/intravenous immunoglobulin; ATG, anti-thymocyte globulin. MFI, median fluorescent intensity.
Measurement of the Anti-ABO Antibody Titer
Anti-ABO antibody titers were determined using standard serological techniques. Briefly, group A or group B indicator red blood cells were suspended in serial doubling dilutions of the recipient's serum, centrifuged, and analyzed for agglutination. This immediate spin assay was defined as representing immunoglobulin (Ig) M activity. Specimens were then incubated at 37°C for 30 min, washed with normal saline, and antihuman globulin (Coombs) antiserum was added. The specimen was then centrifuged and analyzed for agglutination. This antiglobulin assay was defined as representing IgG activity. The most dilute unequivocally positive reaction was defined as the anti-A or anti-B IgM or IgG antibody titer value in each assay.
Protocol and Indication Biopsy
Ten days after transplantation, we performed a protocol biopsy in patients with stable allograft function. Stable graft function was defined as less than 20% increase in the serum creatinine concentration in the 3 days before biopsy and no increase in the dose of immunosuppressive drugs. An indication biopsy was performed in cases of allografts that showed greater than 20% increase in serum creatinine concentration. A 16-gauge biopsy gun was used under ultrasonic localization. Indirect immunofluorescence staining was performed using monoclonal antibodies against complement protein C4d (Biogenesis, Poole, UK; dilution 1:50) for detecting C4d deposition. C4d positivity was defined as diffuse (>50%) and linear staining of peritubular capillaries. Histopathological diagnosis was made according to the revised Banff 2007 classification.Citation8
Clinical Data and Statistics
The following data were analyzed: (1) demographic and immunological characteristics; (2) ABO-type combination of donor and recipient; (3) anti-ABO antibody titer at baseline, after preparation, and during follow-up; (4) intensity of preparation before KT; (5) complications during follow-up; and (6) allograft function at follow-up. Descriptive values were presented as mean ± SD or median and range.
RESULTS
Comparison of the Baseline Characteristics between the High-Titer and Low-Titer Groups
Eight cases belonged to the high-titer group [1:512 (256–1024)] and another six cases were in the low-titer group [1:96 (32–128)] at baseline. No significant differences were found between the high- and low-titer groups in the age at KT (48.3 ± 9.4 vs. 41.8 ± 10.1 years, p >0.05) and the follow-up duration (8.5 ± 4.9 vs. 11.3 ± 2.8 months, p >0.05). The proportion of male patients did not differ significantly between the high- and low-titer groups [87.5% (7/8) vs. 33.3% (2/6), p >0.05]. The HLA mismatch number was significantly higher in the high-titer group (4.3 ± 1.3 vs. 2.8 ± 0.8, p < 0.05). It was the second transplantation for 12.5% (1/8) of the high-titer group and 33.3% (2/6) of the low-titer group. Half of the high-titer group received a kidney from their spouse, and the other half were living relative donor transplantations. In the low-titer group, only one case was a spousal donor transplantation ().
Table 1. Comparison of the baseline characteristics between the high-titer and low-titer groups
Immunological Test Results
In the high-titer group, no patient showed a high PRA (>50%) or a positive cross-match test. The cross-match test was negative in all patients from the low-titer group as well. Nevertheless, three recipients (low-titer 3, 5, and 6) were sensitized to HLA antigen before KT. In low-titer 3 and 5, the PRA was 66.7% (class II) and 50% (class I), respectively, and those were not donor-specific antibodies. In low-titer patient 6, the PRA was 100% (class II) and it was confirmed as donor-specific HLA antibody by the Luminex method (LIFECODES LifeScreen Deluxe kits, Tepnel, CO, USA). After preparation, the PRA was successfully decreased to below 20% in low-titer patients 3 and 5. In low-titer patient 6, the median fluorescent intensity of donor-specific antibody was strong at baseline, but it decreased to the weak level after preparation (C).
Intensity of Preparation
In the high-titer group, the average number of pretransplant PP/IVIG was 10.5 ± 3.5 times. In contrast, it was 6.0 ± 1.3 times (p = 0.01) in the low-titer group. Posttransplant PP/IVIG was required in the high-titer group (62.5%, 1.6 ± 1.8 times) but not in the low-titer group (). In terms of RTX use, 375 mg/m2 was used in 75% (6/8) of the high-titer group. Among them, two patients (high-titer 1 and 4) whose baseline titer was 1:1024 took RTX twice. High-titer 8, whose titer was also 1:1024, took RTX once and bortezomib twice. Another two patients in the high-titer group took 100 mg/m2 of RTX. In the low-titer group, 375 mg/m2 was used in half of the patients (3/6), and another half took 100 mg/m2. Except for low-titer 6, which showed strong donor-specific anti-HLA antibody, all patients in the low-titer group took a single dose of RTX ().
Table 2. Comparison of the preparation intensity between the high-titer and low-titer groups
Change in B-Cell Counts and Anti-ABO Antibody Titers
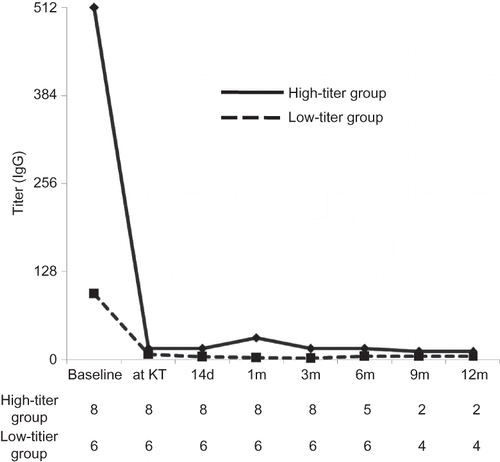
At baseline, CD19- and CD20-positive cell counts were 8.6 ± 5.2%, respectively. In all patients from both groups, CD19- and CD20-positive cells were successfully depleted to below 1% at 14 days after infusion of RTX (). After the scheduled preparation, the antibody titer (IgG/IgM) was successfully decreased to below the target titer (1:32) in both groups. Nonetheless, the median titer at KT was slightly higher in the high-titer group (1:16) compared with the low-titer group (1:8).
Postoperative Recovery Patterns
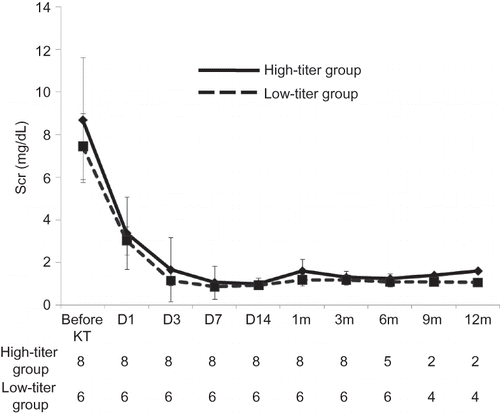
In both groups, all patients showed immediate graft function recovery. Serum creatinine levels at 14 days after KT were 0.93 ± 0.23 mg/dL in the high-titer group and 0.93 ± 0.21 mg/dL in the low-titer group. Except for one patient (high-titer 4), who underwent nephrectomy because of uncontrolled bleeding, graft function was stable at 1 month after KT in both groups (1.21 ± 0.33 mg/dL in both groups) ().
Graft Function during the First 6 Postoperative Months
A total of 11 patients (5 cases from the high-titer group and 6 cases from the low-titer group) were observed for more than 6 months after KT. Serum creatinine level at 6 months after KT was 1.24 ± 0.24 mg/dL in the high-titer group and 1.08 ± 0.24 in the low-titer group (). Except for one patient (low-titer 4) who had taken multiple transfusions with washed red blood cells and fresh frozen plasma because of postoperative bleeding, the anti-ABO antibody titer (IgG) at 6 months after KT was less than 1:16 in all patients ().
Figure 5. Comparison of the change of anti-ABO antibody titer (IgG) between the high-titer and low-titer groups. Represented value is the median level of antibody titer at each period. At baseline, antibody titer was significantly higher in the high-titer group, but the difference significantly decreased at transplantation. Afterward no significant difference was found between two groups until 6 months after KT. We only present IgG level because IgG titer was higher than IgM during entire period.
Note: KT, kidney transplantation.
Protocol Biopsy Findings
Protocol biopsy was performed in eight patients (five cases from the high-titer group and three cases from the low-titer group) who showed stable allograft function after KT and agreed with this procedure. In all cases, morphological changes that can suggest antibody-mediated rejection (AMR) were not detected. The C4d stain was diffusely positive in all three cases in the low-titer group, but only two of five cases from the high-titer group showed diffusely positive C4d (). Indication biopsy was performed three times in two patients, both of whom were in the high-titer group. Their progress is mentioned in the next paragraph.
Table 3. Comparison of the results of protocol biopsy between the high-titer and low-titer groups
Acute Rejection Episodes
There was no acute AMR in either group. Nevertheless, three episodes of acute cellular rejection (ACR) developed in the high-titer group. ACR was treated with 3–5 daily boluses of intravenous methylprednisolone (500 mg/day), followed by a 5–7 day oral steroid taper. In high-titer patient 1, ACR developed 2 months after transplantation and the patient responded well to steroid pulse therapy. In high-titer patient 3, the first ACR developed at 3 months after transplantation and a second episode developed 2 months later. Both episodes also responded well to steroid pulse therapy. According to the Banff classification, the histology stage was ACR Ib in all three biopsies. The C4d stain was diffusely positive in all biopsy specimens, but we could not find any evidence of AMR (). There was no acute rejection episode in the low-titer group.
Table 4. Comparison of complications during follow-up period between the high-titer and low-titer groups
Complications
Two cases of opportunistic viral infection developed in high-titer 1. The first one was CMV and the copy number determined by RT-PCR was 20,724/mm2. It was successfully treated with ganciclovir. The second one was of herpes gingivitis that was cured by acyclovir. Two cases of posttransplant bleeding developed, one in the low-titer group (low-titer 4) and the other in the high-titer group (high-titer 4). It was successfully controlled in low-titer 4. However, in high-titer 4, whose baseline antibody titer was 1:1024, bleeding was not controlled and graft nephrectomy was performed at POD 23 ().
DISCUSSION
The effect of the baseline anti-ABO antibody titer on the graft outcome has been reported with conflicting results. Early reports showed that a high baseline antibody titer is associated with a greater rate of AMR and early graft loss.Citation9,Citation10 However, a recent report showed that a high baseline antibody titer is no longer a significant predictor for graft outcome.Citation11 The results of our study clearly demonstrated that an ABO IKT with a high baseline antibody titer was successful when the antibody titer could be decreased to below 1:32 before KT and suppressed well during the early posttransplant period.
The results of our study showed that the intensity of a preparation regimen was significantly different between the high-titer and low-titer groups. As expected, the patients with a high baseline antibody titer needed more sessions of pretransplant PP/IVIG to reach the target titer.Citation7 In addition, a regimen to deplete B cells or plasma cells was stronger in the patients with a high baseline titer. Previous reports suggested that splenectomy or RTX combined with splenectomy was recommended in patients with a high titer.Citation11–13 In this study, we did not perform splenectomy in all patients with a high titer. Instead, in three patients with a baseline titer of 1:1024, we administered RTX twice, or administered RTX once and bortezomib twice. This finding suggests that a B-cell-depleting antibody can replace splenectomy in patients with a high titer.
It is still controversial whether posttransplant PP/IVIG is needed in ABO IKT. In our study, the rebound of the antibody titer after KT was not significant in the low-titer group. In contrast, five of eight patients in the high-titer group required posttransplant PP/IVIG because of an antibody rebound after KT. This finding suggests that a routine procedure of posttransplant PP/IVIG is not required. Nonetheless, close monitoring of the antibody titer is needed in patients with a high titer because posttransplant PP/IVIG is required in many cases from the high-titer group.
Our results suggest that the critical period in ABO IKT is during the first few weeks after KT. After 2 weeks, the antibody titer did not rise again without PP/IVIG and remained suppressed afterward for several months in both groups. Prolonged suppression of B cells and the antibody titer after RTX infusion has been proved in a previous study.Citation14 This prolonged suppression is important to prevent AMR and enables ABO IKT in the high-titer group. If the antibody titer is well controlled during that period, the risk for AMR can be reduced afterward in the high-titer group. Actually, in spite of the rebound of the antibody titer and requirement for the posttransplant PP/IVIG in the high-titer group, allograft function at 2 weeks after KT was similar between the high-titer and low-titer groups.
In all patients, the peripheral B-cell count was successfully decreased to less than 1% after a single infusion of RTX irrespective of the infused dose. The optimal dose of RTX has not been established in ABO IKT. The most common doseage used to be 375 mg/m2, but this dose was determined for the treatment of malignant B-cell lymphoma, therefore it may not be appropriate in renal transplant recipients.Citation14,Citation15 In this study, the depletion of B cells was successful in patients who took 100 mg/m2 of RTX, which means that a dose reduction to below 375 mg/m2 may be feasible in ABO IKT. Nevertheless, in patients with a baseline titer higher than 1:256, we did not reduce the dose of RTX. Therefore, the effect of a low dose of RTX on those patients with a high titer could not be determined in this study.
A diffuse C4d staining has been accepted as a diagnostic marker for AMR. In ABO IKT, it can develop without any evidence of AMR.Citation8 In addition, C4d positivity was proposed as a marker for accommodation and a less severe immune reaction to the graft kidney in previous reports.Citation16,Citation17 In our study, C4d was diffusely positive in all the biopsies from the low-titer group. In contrast, C4d was diffusely positive in less than half of all patients and other patients showed a focal positive pattern in the high-titer group. It is possible that C4d positivity in the low-titer group suggests a diminished immunological reaction compared with the high-titer group. Nevertheless, this relation between the baseline titer and the C4d positivity could not be determined in this study because of the limited case numbers.
No patient suffered AMR in either group. Nonetheless, three episodes of biopsy-proven ACR developed in two patients from the high-titer group. At the time of acute rejection, the anti-ABO antibody titer was below 1:16 in both patients. Interestingly, both of them were the only two patients with an HLA full mismatch to their donors. The HLA mismatch number is still a significant predictor of ACR in ABO IKT as it is in ABO-compatible KT.Citation18 Therefore, we postulated that not only the high baseline titer but also the HLA full mismatch may be associated with the development of ACR in this study.
Stronger preparation can increase the risk for infectious complications.Citation19,Citation20 No serious infection developed in this study, but one patient with a baseline titer of 1:1024 experienced two opportunistic viral infections. This patient received a stronger preparation including two administrations of RTX and extensive pre- and posttransplant PP/IVIG. In the low-titer group, one patient experienced pyoknee, but this condition developed after a knee joint injury, and she did not receive appropriate treatment for several days. Hence, it should not be regarded as an opportunistic infection. Our results suggest that despite close monitoring and prophylaxis, opportunistic infections can develop because of strong immunosuppression in the high-titer group.
Postoperative bleeding developed in two patients, one from the low-titer group and another from the high-titer group. In the low-titer group patient, bleeding around the graft kidney developed 7 days after KT. After hematoma removal and bleeding control, no bleeding was detected anymore and the progress was favorable. The cause of the hematoma was blood oozing induced by thrombocytopenia. In the patient from the high-titer group, the baseline titer was 1:1024. PP/IVIG was performed 15 times pre- and 6 times posttransplantation. Bleeding around the graft kidney developed 3 days after the protocol biopsy, and graft nephrectomy was performed because it could not be controlled by operation. We assumed that massive pre- and posttransplant PP/IVIG may increase the bleeding tendency, which causes severe bleeding at the biopsy site. We suggest that in the high-titer patients, especially those who underwent massive PP/IVIG, an invasive procedure should be avoided because of the increased risk for bleeding.
In summary, patients with a high baseline titer, ABO IKT can be performed without increased risk of AMR if the antibody titer is controlled well during the early posttransplant period. However, adverse events such as opportunistic infections and bleeding can increase because of more intensive preparation. This may be an important obstacle for a successful transplantation in these patients.
ACKNOWLEDGMENT
This study was supported by a grant (A092258) from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Korean Network for Organ Sharing. KONOS 2009 annual report. Available at www.konons.go.kr. Accessed August 21, 2010.
- Huh KH, Kim MS, Ju MK, Exchange living-donor kidney transplantation: Merits and limitations. Transplantation. 2008;86:430–435.
- Hume DM, Merrill JP, Miller BF, Thorn GW. Experiences with renal homotransplantation in the human: Report of nine cases. J Clin Invest. 1955;34:327–382.
- Alexandre GP, De Bruyere M, Squifflet JP, Moriau M, Latinne D, Pirson Y. Human ABO-incompatible living donor renal homografts. Neth J Med. 1985;28:231–234.
- Rahman T, Harper L. Plasmapheresis in nephrology: An update. Curr Opin Nephrol Hypertens. 2006;15:603–609.
- Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: Integrating paired donation into desensitization protocols. Am J Transplant. 2010;10:449–457.
- Tobian AA, Shirey RS, Montgomery RA, Tisch DJ, Ness PM, King KE. Therapeutic plasma exchange reduces ABO titers to permit ABO-incompatible renal transplantation. Transfusion. 2009;49:1248–1254.
- Solez K, Colvin RB, Racusen LC, Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–760.
- Gloor JM, Lager DJ, Moore SB, ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation. 2003;75:971–977.
- Shimmura H, Tanabe K, Ishikawa N, Tokumoto T, Takahashi K, Toma H. Role of anti-A/B antibody titers in results of ABO-incompatible kidney transplantation. Transplantation. 2000;70:1331–1335.
- Shimmura H, Tanabe K, Ishida H, Lack of correlation between results of ABO-incompatible living kidney transplantation and anti-ABO blood type antibody titers under our current immunosuppression. Transplantation. 2005;80:985–988.
- Uchida J, Iwai T, Kato M, A novel approach to successful ABO-incompatible high-titer renal transplantation. Transplant Proc. 2008;40:2285–2288.
- Gloor JM, Lager DJ, Fidler ME, A comparison of splenectomy versus intensive posttransplant antidonor blood group antibody monitoring without splenectomy in ABO-incompatible kidney transplantation. Transplantation. 2005;80:1572–1577.
- Vieira CA, Agarwal A, Book BK, Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: 1. Safety, pharmacodynamics, and pharmacokinetics. Transplantation. 2004;77:542–548.
- Toki D, Ishida H, Horita S, Setoguchi K, Yamaguchi Y, Tanabe K. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int. 2009;22:447–454.
- Setoguchi K, Ishida H, Shimmura H, Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am J Transplant. 2008;8:86–94.
- Haas M, Segev DL, Racusen LC, C4d deposition without rejection correlates with reduced early scarring in ABO-incompatible renal allografts. J Am Soc Nephrol. 2009;20:197–204.
- Shimmura H, Tanabe K, Tokumoto T, Impact of HLA-identity on results of ABO-incompatible living kidney transplantation. Transplant Proc. 2004;36:2172–2174.
- Grim SA, Pham T, Thielke J, Infectious complications associated with the use of rituximab for ABO-incompatible and positive cross-match renal transplant recipients. Clin Transplant. 2007;21:628–632.
- Aikawa A, Ohara T, Arai K, Clinical outcome and accommodation in ABO incompatible kidney transplantation. Clin Transplant. 2004;1:135–142.