Abstract
The stimulus for caspase-mediated renal cell apoptosis in septic acute renal failure (ARF) is unclear. To demonstrate the nephrotoxic effects of bacterial cell wall components, the anti-cellular activity of bacterial muropeptides (muramyl dipeptides), peptidoglycans, and lipopolysaccharides was investigated in rabbit kidney cells. Changes in the cell membrane (APOPercentage™ dye uptake), caspase activities, and DNA degradation were quantified colorimetrically and using densitometric assays and their inhibition by caspase-specific and pan-caspase inhibitors was determined. The onset and levels of APOPercentage™ dye-positive rabbit kidney cells, caspase activities, and DNA degradation were closely associated. Specific caspase-1, -2, -3, -4, -8, -10, and -12 inhibitors reduced caspase-3 activity by ≥40%, but only caspase-3 and -8-specific inhibitors reduced apoptotic DNA levels. Pan-caspase inhibitor Q-VD-OPh was 10-fold more effective at inhibiting rabbit kidney cell death, caspase activation, and DNA degradation than caspase-family inhibitor Z-VAD-FMK. Apoptosis was inhibited effectively by both pan-caspase inhibitors when applied early during the stimulus-to-response period. Multiple initiator and effector caspases were activated suggesting extrinsic, intrinsic, and endoplasmic reticulum/stress apoptotic pathway stimulation in rabbit kidney cells treated with bacterial cell wall components. The results provide in vitro support for bacterial cell wall-induced apoptosis as a pathogenic mechanism of renal cell death in septic ARF and support the potential prophylactic use of pan-caspase inhibitors to suppress septic ARF.
INTRODUCTION
Acute and chronic bacterial infection can affect renal function significantly and, vice versa, diminished renal function can profoundly affect the course of acute and chronic bacterial infection. Accordingly, multiple mechanisms have been proposed for the hyper-inflammation and apoptosis associated with septic shock and acute renal failure (ARF).Citation1–8 That is, bacteria can stimulate the extrinsic apoptotic pathway in leukocytes and/or renal cells via interactions with death receptors leading to Fas-associated death domain (FADD) activation followed by proteolytic activation of inflammatory cytokines and caspases leading to cell death. Also, bacteria can stimulate intrinsic apoptotic pathways via release of apoptogenic factors that affect Ca2+ levels in endoplasmic reticulum (ER) and mitochondria leading to activation of caspase and cell death. Further, bacterial infection may cause cell death via non-apoptotic pathways, such as necrosis and autophagy.Citation9–12
The extrinsic and intrinsic apoptotic pathways ultimately converge to activate caspase. Caspases are constitutively expressed pro-enzymes that are activated by proteolysis and exert specific cysteine protease activity. In addition to their normal physiological roles in cell growth and terminal differentiation, caspases are the effector molecules of apoptosis.Citation10–12 Three major caspase groups may be activated during apoptosis: initiator caspase (caspase-2, -8, -9, and -10), effector/executioner caspase (caspase-3, -6, and -7), and inflammatory caspase (caspase-1, -4, -5, -11, -13, and -14).Citation10–13 Initiator/inflammatory caspases activate the executioner caspase that cleaves specific protein targets resulting in the cellular and biochemical changes associated with cell death such as cell shrinkage, membrane blebbing/degradation, and nuclear fragmentation and degradation. Thus, the caspases induced in tissues by bacteria during sepsis represents a unique therapeutic target.Citation12,Citation13
The pathogenesis of sepsis-induced renal dysfunction is controversial. The emerging concept suggests that septic ARF is likely an immune or toxic state (i.e., acute tubule apoptosis) rather than a hemodynamic condition (i.e., acute tubule necrosis).Citation14 The likelihood that renal tubule cell apoptosis is induced by bacterial cell wall components is supported by the acute changes in rabbit renal function induced by intravenous injection of muramyl dipeptide (MDP) and endotoxin.Citation15–17 Moreover, MDP (the smallest biologically active component of gram-positive and gram-negative bacteria cell wall peptidoglycan), peptidoglycan (PGN), and lipopoly- saccharide (LPS) induce apoptosis in γ-glutamyl transpeptidase (GGT)-positive rabbit RK13 renal epithelial cells,Citation18 as well as renal tubule cell lines of other mammalian species.Citation17 MDP and PGN induce rabbit kidney cell apoptosis via Ca2+-dependent interactions with surface calreticulin (CRT)Citation19 followed by FADD stimulation and effector caspase-3 activityCitation20 (CRT is a multi-functional Ca2+-binding chaperone protein in the ER).Citation21 In contrast, LPS-induced, FADD-dependent renal cell apoptosis is death receptor-independent and requires high LPS concentrations and a longer incubation period.Citation17,Citation20 LPS increases the permeability of the proximal tubules, increases Ca2+ in mitochondria, and induces mitochondrial structural damage and caspase-mediated apoptosis.Citation1–3,Citation22 Further, endotoxin-induced inflammatory cytokines and apoptosis were inhibited by pan-caspase inhibitors suggesting that apoptotic cells may be the source of the inflammation in ARF.Citation2,Citation3
The caspases induced in rabbit kidney cells by bacteria or bacterial cell wall components in vitro or in vivo have not been identified, but their induction is suggested based upon the observation that inhibition of caspase increases survival and renal function in septic and ARF models.Citation2,Citation3,Citation13 This study investigated the caspases induced by bacterial cell wall components in rabbit kidney cells and demonstrates the anti-apoptogenic effects of individual and broad-spectrum caspase inhibitors against kidney cell death, caspase-3 activation, and DNA degradation. The results are consistent with bacterial cell wall stimulation of multiple caspase and apoptotic pathways in rabbit kidney cells, provide an insight as to why pan-caspase inhibition suppresses ARF, and support bacterial cell wall-induced renal cell apoptosis as a plausible if not critical pathogenic mechanism in ARF.
MATERIALS AND METHODS
Cells and Tissue Culture
CRT- and GGT-positive, tubule-like rabbit kidney (RK13) cells (American Type Culture Collection; CCL37) are highly sensitive to the apoptogenic effects of MDP, PGN, and LPS.Citation17,Citation20 RK13 cells were cultured in Dulbecco's minimum essential medium with 10% bovine calf serum (DMEM) (Sigma, St. Louis, MO, USA) as previously reported.Citation17 For experimental procedures, trypsinized cells were suspended in DMEM (1 × 10Citation6 cells/mL) and pipetted into glass slide cultures (Lab-Tek® Chamber SlideTM, 8 chambers/slide; Nalge Nunc International, Naperville, IL, USA) or 6-well culture dish plates (2 mL/culture) (Nalge Nunc International). The cultures were incubated for 48–72 h at 37°C in a water-jacketed incubator in 5% humidified CO2 atmosphere (Forma Scientific, Marietta, OH, USA) before use.
Bacterial Reagents
Muropeptides [N-acetylmuramyl-l-alanyl-d-isoglutamine (MDP), N-acetylmuramyl-l-alanyl-l-isoglutamine (L,L-MDP), N-acetylmuramyl-d-alanyl-d-isoglutamine (D,D-MDP), 4-(2-acetamido-2-deoxy-β-d-gluco-pyranosyl) N-acetylmuramyl-l-alanyl-d-glutamate amide (ADG-MDP), and N-acetylmuramyl-6-0-stearoyl-l-alanyl-d-glutamine amide (MSDP)] were purchased from Sigma Biological Company and diluted in phosphate-buffered saline (PBS; pH 7.4) (1 mg/mL), filter sterilized, aliquoted, and stored at −20°C. Stock PGN suspensions (1 mg/mL) of Bacillus subtilis, Micrococcus luteus, Methanobacterium sp., Streptomyces sp., Staphylococcus aureus, and Saccharomyces cerevisiae (Fluka; [email protected]) (Sigma) were made in PBS. Escherichia coli and Klebsiella pneumoniae LPS (Sigma) were dissolved in PBS (10 mg/mL). Crude Helicobacter pylori cell wall was prepared by sonication of H. pylori suspended in 0.9% NaCl followed by centrifugation (1000 × g for 10 min). The cell walls in the harvested supernatant were diluted to a concentration of 1 mg/mL in PBS and frozen at −20°C until use. Staurosporine (STP; a product of Streptomyces with broad-spectrum kinase inhibition activity that induces necrosis and caspase-dependent cell death)Citation23 was employed as a control. The concentration of MDP, PGN, and LPS that contained a 50% tissue culture apoptotic dose (TCAD50) was determined as described previously.Citation17
Apoptosis Induction
The methods for induction of apoptosis by MDP, PGN, LPS, and STP in RK13 cells, quantification of caspase activity, and apoptotic DNA ladders were described previously.Citation17,Citation20 Briefly, MDPs (1 µg/mL), PGN (200 µg/mL), LPS (500 µg/mL), or STP (1 µg/mL) was added to the medium of triplicate cultures of a 6-well tissue culture plate (Sarstedt, Newton, NC, USA) and incubated at 37°C in a 5% humidified atmosphere. The medium was removed from cultures hourly through 8 h, or after 12, 18, and 24 h incubation. The cells were rinsed twice with cold PBS and 200 µL of cell lysis buffer (250 mM HEPES, pH 7.4, 25 mM CHAPS, 25 mM DTT) (Caspase Assay Kit; Sigma) was added per 6-well plate culture. The RK13 cells were scrapped from the tissue culture dish using a Teflon spatula, the cell lysis buffer harvested into capped centrifuge tubes, and frozen at −20°C.
Caspase and Interleukin-1β Detection
Rabbit polyclonal antibody (#16745; Cayman Chemical Comp., Ann Arbor, MI, USA) and monoclonal antibody (#9668; Cell Signaling Technology, Inc., Danvers, MA, USA) to human caspase-3 and human interleukin-1β (IL-1β) (NB600-1381; Novus Biological, Littleton, CO, USA) (caspase-1 cleaves IL-1βCitation24) were used to detect caspase-3 and IL-1β, respectively, in RK13 cells by the indirect fluorescent antibody (IFA) method.Citation25 Briefly, 4% p-formaldehyde-fixed RK13 cells on glass slides (Lab-Tek® chamber slide systems; Nunc Nalgene International, Rochester, NY, USA) were reacted with primary antibodies (1:200 dilution in PBS) overnight at 4°C. Secondary fluorescein-isothiocyanate (FITC)-tagged goat anti-rabbit IgG (Fab) (Cappel, ICN Biomedicals, Inc., Aurora, OH, USA) (200 µL/glass slide chamber of a 1:500 dilution in PBS) was applied for 6 h. Cytological changes and IFA staining were observed by a Nikon Eclipse TE300 inverted microscope with an epi-fluorescence attachment and a single band exciter for FITC (492 nm/18×). Digital files were made with a Photometrics Cool SNAPfx monochrome CCD camera using Scanalytics IPLab software.
FLICA-Apoptosis Detection Assay
Fluorescein-tagged (carboxyfluorescein) generic caspase probe FLICA-FAM-VAD-FMK (FLICA-Apoptosis Detection Kit Caspase Assay, Immunochemistry Technologies, LLC., Bloomington, MN, USA) irreversibly binds to activated caspase-1, -3, -4, -5, -6, -8, and -9 and was used to detect activated caspase in unfixed apoptotic RK13 cells at 6 h post-MDP treatment. The assay was performed according to the manufacturer's instruction. FLICA staining was observed and digital image files were obtained as described for IFA.
Caspase Assay Reagents
Three p-nitoanalide-tagged caspase-1 substrates, two caspase-2 substrates, and a single substrate specific for caspase-3, -6, -8, -9, and -10 () were purchased from Sigma, BioVision, Inc. (Mountain View, CA, USA) or AnaSpec, Inc. (San Jose, CA, USA) and dissolved in DMSO (2 mM). Cell-permeable fluoromethyl ketone (FMK)-linked oligopeptide (Z-R-FMK) inhibitors of caspase-1, -2, -3, -4, -5, -6, -8, -9, -10, -12, and -13 (Caspase-Family Inhibitor Set IV, BioVision) () were dissolved in water or DMSO (1 mM) as per the manufacturer's instructions and stored at −20°C. Caspase-family inhibitor Z-VAD-FMK (irreversibly binds to caspase-1, -3, -4, -5, -6, -7, -8, and -9), FMK control (BioVision), and the broad-spectrum caspase inhibitor InSolutionTM Q-VD-OPh [quinolyl-valyl-O-methylaspartyl-(-2,6-difluorophenoxy)-methyl ketone] (Calbiochem/EMD Chemicals, Inc., Darmstadt, Germany) were dissolved in DMSO (1 mM) and stored at −20°C.
Table 1. Caspase-specific substrates for colorimetric quantification and 50% inhibitory dose (ID50) against MDP-induced caspase-3 activity
Quantification of Cell Death, Caspase Activity, and DNA Degradation
Early apoptotic cell membrane/transport changes were detected using the APOPercentage™ Apoptosis Assay kit (Biocolor Ltd., Newtonabbey, Northern Ireland; www.biocolor.co.uk) as previously described.Citation20 Briefly, the culture medium was removed from replicate control and MDP-treated RK13 cells in 6-well dish cultures before 1 mL of pre-warmed (37°C) DMEM containing APOPercentageTM dye (25 µL of dye/0.5 mL) was added to each culture. After incubation at 37°C for 30 min in accordance with the manufacturer's instructions, the cells were washed five times with warm PBS, viewed microscopically, and 300 µL of Dye Releasing Reagent was applied. The amounts of dye taken up by the cells and released into the releasing reagent were measured spectrophotometrically at OD570 and the average OD and percent differences between dye uptake in control and MDP-treated cultures were calculated.
To assay for caspase,Citation25 the frozen cell lysis buffer samples were thawed and centrifuged at 10,000 × g for 10 min in desktop Eppendorf microcentrifuge. Ten microliters of each sample were added per replicate 96-well microtiter plate well (Starstedt) containing 100 µL of caspase assay buffer (50 mM HEPES, pH 7.4, 0.1% CHAPS, 10 mM DTT, 100 mM NaCl, 1 mM EDTA, and 10% sucrose) and 1 µM of p-nitoanalide (pNA)-tagged caspase-1, -2, -3, -6, -8, -9, or -10 -specific substrates (1 µM) () as per the manufacturer's instructions (Caspase Assay Kit; Sigma). The activity was measured spectrophotometrically (OD405) after 3 h incubation at 37°C. The mean caspase activity was calculated and expressed in units. One unit of caspase activity equals 1.0 nmol of caspase-specific substrate p-nitroaniline formed per hour per milligram or per hour per milliliter. The protein concentration in clarified lysates was determined by Bio-Rad Protein assay (Bio-Rad, Hercules, CA, USA).
Apoptotic DNA ladders (DNA degradation by endonucleases) were detected by adding DNA extraction buffer (300 µL; 50 mM Tris-Cl, pH 7.5, 10 mM EDTA, 0.5% Triton X-100, 0.5% NP-40) to the cell pellets of each sample used for caspase analysis. The DNA was phenol extracted and endonuclease cleavage fragments of DNA were separated by agarose gel electrophoresis as described previously.Citation17 Digital images of ethidium bromide binding extracted cellular DNA and molecular weight standard (Gene Mate; Quanti-Marker 100 bp;[email protected]) were captured using the Bio-Rad Universal Hood and Quantity One® 1-D Analysis Software (Bio-Rad Laboratories; www.bio-rad.com). Densitometric analysis of bands of degraded cellular DNA was performed using Image J 1.41o (http://rsb.info.nih.gov/i) software.
Western Blot Analysis
Western blot analyses for detection of caspase-3, -6, -7, -10, and -12 were performed as reported.Citation25 Briefly, monoclonal antibody (#9668; Cell Signaling Technology, Inc.) to human caspase-3, rabbit polyclonal antibodies against human caspases-6 (#9762) and -7 (#9492; Cell Signaling Technology, Inc.), human IL-1β (NB600-1381; Novus Biological, Littleton, CO, USA) (caspase-1 cleaves IL-1β),Citation24 caspase-10 (ab2012), and caspase-12 (ab62484; Abcam, Cambridge, MA, USA) were diluted in PBS and incubated with transblotted proteins on nitrocellulose membranes (Pierce Biotechnology, Inc., Rockford, IL, USA) in accordance with the manufacturer's recommendations for western blot analysis. The immunoreactive bands were detected using horseradish peroxidase-tagged secondary antibodies to rabbit IgG (Fab2 fragment; Jackson ImmununiResearch Laboratories, Inc., West Grove, PA, USA). Digital image files were created and densitometric analyses of immunoreactive protein bands were performed as above.
Caspase-Specific and Pan-caspase Inhibition of Kidney Cell Apoptosis
To determine the relative role of each caspase in MDP-induced rabbit kidney cell apoptosis, replicate RK13 cell 6-well plate cultures were incubated in medium containing serially diluted caspase-1, -2, -3, -4, -5, -6, -8, - 9, -10, -12, and -13 substrate-specific inhibitors, as well as caspase-family inhibitors Z-VAD-FMK and pan-caspase inhibitor Q-VD-OPh for 6 h. The cells were harvested and processed for detection of caspase-3 and apoptotic DNA ladders.Citation17,Citation20 Caspase inhibitor anti-apoptogenicity was measured as the percent caspase-3 inhibition and/or reduction in densitometric levels of apoptotic DNA ladders detected in treated versus untreated cultures.
To assess the anti-apoptogenicity of the pan-caspase inhibitors against apoptosis induced by different bacterial cell wall components, replicate RK13 cell cultures were treated for 1 h with fresh media containing 2 µM of the pan-caspase inhibitors prior to the addition of MDP (0.2 µg/mL), PGN (200 µg/mL), LPS (500 µg/mL), or STP (1 µg/mL). After incubation with MDP (6 h), PGNs (8 h), STP (12 h), or LPS (24 h) (generally 30−50% of cells exhibited microscopic signs of apoptosis), the medium was removed from the culture and the cells were rinsed three times gently with 1 mL cold PBS. Caspase lysis buffer (200 µL) was added to each 6-well plate culture, and the cell lysates were harvested into microcentrifuge tubes, centrifuged (10,000 × g for 10 min), and caspase-3 activity quantified in the supernatant. Apoptotic DNA degradation was detected and quantified as above.
Dose- and Time-Dependent Inhibition by Caspase Inhibitors
Dose- and time-dependent inhibition studies of caspase-specific and pan-caspase inhibitors against MDP-induced apoptosis (caspase-3 activation and DNA degradation) were performed using similar methods as above. For dose-dependent studies, half-log10 dilutions (1:3.2) of caspase-specific and pan-caspase inhibitors (Q-VD-OPh and Z-VAD-FMK) in DMEM were pipetted into replicate RK13 cell cultures pre-treated with MDP for 30 min and then incubated for 6 h. The medium was removed, the cells were rinsed three times with 1 mL cold PBS, and 200 µL of cell lysis buffer was added per culture. The cell lysates were collected, clarified by centrifugation (10,000 × g/5 min), and assayed for caspase-3 activity. The percent caspase inhibition was calculated by dividing the caspase activity in inhibitor-treated cultures minus the caspase activity in control by the caspase activity in MDP-treated cultures minus the caspase-3 activity in control cultures × 100. The mean 50% inhibitory dose (ID50) was calculated from the results of three independent experiments. Using the same sample, DNA was extracted from the caspase lysate/cell pellet and processed for detection of apoptotic DNA degradation and quantification by densitometric analysis. For time-dependent inhibition studies, pan-caspase inhibitors were added or removed at times post-MDP challenge. For time of addition studies, replicate RK13 cell cultures were incubated for 30 min in media containing 1 µg/mL MDP. The MDP medium was removed and the cells were rinsed three times with 1 mL of pre-warmed DMEM. Fresh media containing Z-VAD-FMK (5 µM) or Q-VD-OPh (1 µM) was added to the culture medium at 0, 1, 2, 3, 4, and 5 h. Each experiment was terminated at 6 h post-MDP challenge and the cells processed for detection and quantification of caspase activity and apoptotic DNA degradation. For time of removal studies, replicate RK13 cell cultures were treated with 1 µg/mL MDP for 30 min. The MDP was removed, and the cells were rinsed with fresh DMEM. Z-VAD-FMK (5 µM) or Q-VD-OPh (1 µM) in DMEM was pipetted into replicate cultures. The media containing the caspase inhibitor was removed from cultures after 0.5, 1, 1.5, 2, 3, and 4 h incubation. The cells were washed three times with 1 mL pre-warmed media, and 1.0 mL fresh pre-warmed DMEM added to the cell cultures. The experiments were terminated after 6 h incubation and the cell lysates prepared and assayed for caspase and DNA degradation.
Statistical Analysis
Data were reported as mean ± standard deviation. Data were analyzed using a Dell PC/Microsoft Excel statistical software program. Data comparing two groups were analyzed using Student's t-test. Significance was accepted at p ≤ 0.05.
RESULTS
Caspase-Mediated Apoptosis Induced by MDP and PGN in RK13 Cells
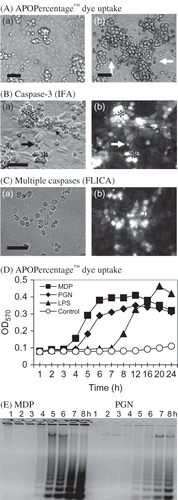
Tissue culture medium containing 1 µg/mL MDP [200 tissue culture apoptotic doses (TCAD)50/mL]Citation17 was pipetted into RK13 cell cultures. The cultures were incubated at 37°C in a humidified 5% CO2 atmosphere for 30 min before the medium was removed, the cells rinsed three times with 1 mL volumes of PBS, and fresh DMEM pipetted into each culture and incubated at 37°C. Early cellular changes characteristic of apoptotic cell death such as increased light refraction, rounding, nuclear condensation, APOPercentage™ dye staining (Ab), nuclear fragmentation (cell bodies), and membrane blebbing were observed 4–5 h post-incubation with 1 µM MDP (Ba and b) [5–6 h post 200 µg PGN/mL (not shown)]. Immunoreactive caspase-3 was detected at 5 h post-MDP in p-formaldehyde-fixed apoptotic RK13 cells (Bb). Concomitantly, multiple caspase activation in apoptotic RK13 cells (Ca) was suggested by staining with FLICA-FAM-VAD-FMK (Cb). The number of cells exhibiting APOPercentage™ dye uptake increased through 24 h in MDP-, PGN-, and LPS-treated RK13 cell cultures (D). Significant dye uptake by cells in MDP-treated cultures was detected earlier in MDP-treated culture than in PGN- and LPS-treated cultures. The apoptotic cell phenotype (Ba and Ca) was closely associated with detection of DNA degradation in RK13 cells treated with MDP and PGN (200 µg/mL continuously) (10 TCAD50/mL) (E). Note that apoptotic DNA degradation (ladders) was detectable in cells harvested at 4 h post-MDP treatment or 5 h post-PGN treatment and the amount of DNA degradation continued to increase through 8 h (E). The results support the time-dependent apoptogenicity of MDP and PGN in rabbit kidney cells. Further, the results suggest an association between the number of dye-positive apoptotic cells, and levels of caspase-3 activity and DNA degradation in MDP/PGN-treated RK13 cell cultures. The positive FLICA-FAM-VAD-FMK reaction suggests activation of multiple caspases in apoptotic RK13 cells.
Figure 1. Induction of acute rabbit kidney cell death. (A) Micrographs showing APOPercentage™ staining of (a) untreated control and (b) MDP-treated RK13 cells. (B) Micrographs showing p-formaldehyde-fixed (a) apoptotic RK13 cells (*), membrane blebbing (small arrow), and apoptotic cell bodies (large arrows); (b) positive for caspase-3 in MDP-treated RK13 cells after 5 h incubation. (C) Micrographs showing (a) apoptotic RK13 cells positive for (b) FLICA-FAM-VAD-FMK (carboxyfluorescein-tagged pan-caspase substrate) (bar = 50 µm). (D) Time course of APOPercentage™ dye uptake in control and MDP, Streptomyces spp. PGN, and E. coli LPS-treated RK13 cell cultures. (Each point is the mean OD570 from two cultures.) (E) Detection of apoptotic RK13 cell DNA ladders (indicative of endonuclease activation) at hourly time intervals post-MDP and Streptomyces spp. PGN treatment.
Multiple Caspases Activated by Different MDP, PGN, and LPS in RK13 Cells
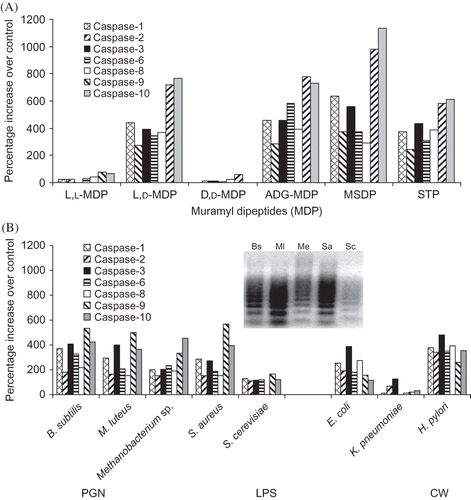
The following experiments were performed to compare the caspases induced by different muropeptides, as well as different bacterial components (i.e., PGN, LPS, and STP). Rabbit RK13 cells were incubated with L,L-MDP; MDP; D,D-MDP; ADG-MDP; and MSDP (2 µg/mL) for 6 h, STP (5 µg/mL) for 8 h, or PGNs from B. subtilis, M. luteus, Methanobacterium spp., S. aureus, or S. cerevisiae (200 µg/mL) up to 24 h (). Caspase-1 (ICE), -2 (ICH), -3, -6, -8, -9, and -10 activities were generally >200% higher in MDP, ADG-MDP, and MSDP than in control, but not in L,L-MDP- and D,D-MDP-treated RK13 cells (A), consistent with the previously reported non-apoptogenic activity of the L,L-MDP and D,D-MDP.Citation17 These results support the stereo-isomer-specific apoptogenicity of MDP containing l-alanyl-d-isoglutamine and suggest apoptogenic muropeptides induce similar caspase cascades in RK13 cells. Similarly, STP (a potent apoptogenic agent) increased multiple caspase activities by >200%. In comparison, PGN from B. subtilis, M. luteus, Methanobacterium spp., and S. aureus, but not S. cerevisiae increased caspase activity by ≥150% over RK13 cells control following 16–24 h incubation (B). Note, caspase-1 (ICE), -2 (ICH), -3, -6, and -8 activities were generally higher in RK13 cell cultures treated with PGN from B. subtilis, M. luteus, Methanobacterium spp., S. aureus, and Streptomyces spp. (see C) than in the RK13 cell control and S. cerevisiae PGN-treated cultures. The percent increases in caspase activities were higher in H. pylori and E. coli than in K. pneumoniae LPS-treated RK13 cells. Degraded DNA levels appeared to parallel caspase activity levels in PGN- (B; inset), MDP- (C), and LPS-treated RK13 cells. The results suggest that only muropeptides containing l-alanyl-d-isoglutamine, but most PGNs and LPSs from different bacteria and STP induce multiple caspases. The results suggest that the substrate specificities of the rabbit caspase parallel the substrate specificities of murine and human caspase.Citation9 Activation of multiple initiator, inflammatory, and stress caspases suggests stimulation of extrinsic and intrinsic apoptotic pathways.
Figure 2. Caspase activities in apoptotic rabbit kidney cells (A) at 6 h post-treatment with MDPs or STP, (B) at 12 h post-treatment with PGNs (200 µg/mL) from B. subtilis (Bs), M. luteus (Ml), Methanobacterium spp. (Me), S. aureus (Sa), and S. cerevisiae (Sc) and 24 h post-treatment with LPS (500 µg/mL) from E. coli, K. pneumoniae, and H. pylori. Mean results of two experiments (two cultures/experiment) assayed in duplicate. (Inset: Apoptotic DNA ladders in RK13 cells treated with Bs, Ml, Me, Sa, and Sc PGNs.)
Time-Dependent Activation of Caspase in Apoptotic RK13 Cells
The time course of each caspase was determined to assess the activation sequence in MDP- and PGN-treated RK13 cells (). Lysates from MDP-treated RK13 cells collected after 3–4 h incubation contained >twofold higher caspase-1 (ICE), -2 (ICH), -3, -6, -8, and -10 activities than the control lysates (A). Caspase levels increased through 8 h and remained essentially constant through 12 h post-MDP. Caspase-9 and -10 were detected at 5–6 h post-MDP and increased through 12 h. Similarly, caspase-1, -2, -3, -6, -8, -9, and -10 were increased in RK13 cell lysates harvested after 4–8 h incubation in medium containing PGN (Streptomyces sp.) (B). Maximal caspase-3 activity was detected in MDP- and PGN-treated cultures at 6 h (163 ± 34 and 128 ± 29 U/mg/culture, respectively) (A and B). Although caspase and apoptotic DNA degradation (C) were generally higher in MDP-treated than in PGN-treated RK13 cells, the relative caspase levels were similar, with PGN-induced caspase-2 being the possible exception. The results of the induction studies suggest that MDP- and PGN-induced cell death is mediated by multiple initiator caspase (-1, -2, -8, -9, and -10) and effector caspase-3 and -6. Concomitantly, western blot analyses supported activation of rabbit caspase-3 followed closely by caspase-6 and probably caspase-7 activation (apoptosome formation). That is, cleaved caspase-3 immunoreactive proteins were detected in RK13 cell lysates collected at 4–8 h post-MDP treatment consistent with caspase-3 activation (i.e., cleavage of 32 kDa pro-caspase-3 yielding 18 kDa) (C). Three immunoreactive caspase-6 proteins (37, 39, and 31 kDa) were detected in control and MDP-treated RK13 cells up to 4 h post-MDP treatment (D). The 37 kDa protein disappeared and the 31 kDa protein was reduced (by 85% at 8 h) as a 14 kDa caspase-6 immunoreactive protein appeared at 5 h post-MDP. An increase (1.7–2.3-fold increase by densitometry) in a caspase-7 immunoreactive protein (26 kDa) was detected in lysates collected at 5–8 h (in two of three experiments) (E). As caspase-1 activity was increased in rabbit kidney cells and it cleaves pro-inflammatory IL-1β,Citation24 it was of interest to assess IL-1β levels in apoptotic rabbit kidney cells. Modest increases in IL-1β were detected by western blot analysis (≤25% increase) (F) and by IFA (Gb and d) after 4–6 h MDP treatment suggesting the possible cleavage of rabbit IL-1β, but immunoreactive cleaved IL-1β was not detected. The western blot analysis results support the time course of MDP-induced caspase-3 and -6 and probably caspase-7 activity (constitute an apoptosome) in apoptotic RK13 cells (). In contrast, immunoreactive caspase-10 and -12 proteins were not detectable in rabbit kidney cells or lysates (not shown). Western blots of cell lysates harvested from PGN-treated RK13 cells incubated for 8 h yielded caspase-3, -6, -7, and IL-1β profiles similar to MDP (not shown). The failure to detect caspase-10 and -12 and cleaved IL-1β may have been due to the failure of monoclonal antibodies to bind efficiently under western blot conditions. The results support the utility of caspase-specific substrates in detection of rabbit caspase. Overall, the results are consistent with the idea that similar cascades were induced by MDP and PGN in RK13 cells suggesting the possible stimulation of extrinsic and intrinsic apoptotic pathways.
Figure 3. Quantification of caspase activities at times post (A) MDP and (B) PGN. (Each point represents the mean of three experiments assayed in duplicate for each caspase activity.) Western blot analysis for cleaved rabbit (C) caspase-3 (<15 kDa; arrow); (D) caspase-6 (<15 kDa; arrow); (E) caspase-7 (28 kDa; arrow); and (F) IL-1β (32 kDa; arrow) immunoreactive proteins in control and MDP-treated rabbit kidney RK13 cells at times post-treatment. (G) IFA detection of immunoreactive IL-1β in p-formaldehyde fixed control (a, b) and MDP-treated (c, d) RK13 cells at 6 h post-incubation.
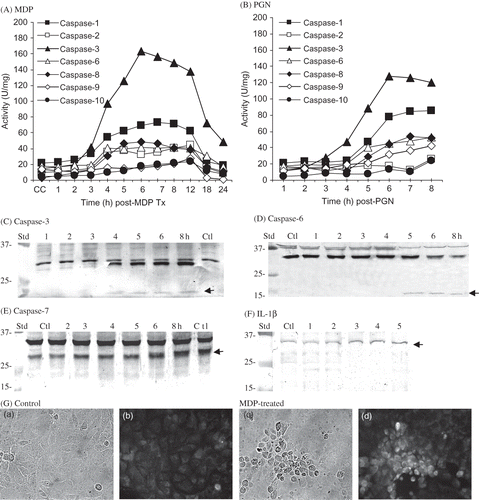
Inhibition of MDP-Induced Apoptosis by Caspase-Specific and Pan-caspase Inhibitors
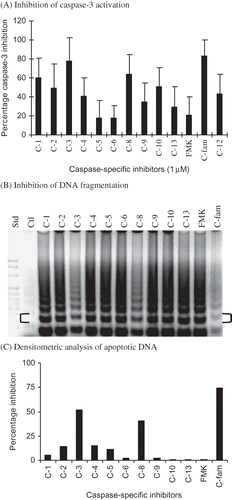
To assess the relative role of each caspase detected, the inhibitory activity of 1 µM of caspase-specific peptide inhibitor of caspase-1, -2, -3, -4, -5, -6, -8, -9, -10, -12, and -13 and the pan-caspase inhibitors against MDP-induced apoptosis was determined (). Substrate-specific inhibitors of caspase-1, -2, -4, -8, -10, -12, and caspase-family (Z-VAD-FMK) inhibited caspase-3 activity by ≥40% (p-values < 0.02) (A) suggesting they are upstream of caspase-3 activation. Caspase-3 inhibition by substrate-specific inhibitors of caspase-5, -6, -9, -10, and -13 was not significantly different (p-values ≥ 0.2) from FMK-treated placebo control cultures. Substrate-specific inhibition of caspase-5 and -6 was least effective in preventing caspase-3 activation. [It should be noted that significant cross-caspase inhibition was not detected for the caspase-specific inhibitors against the rabbit caspase in MDP-induced apoptotic RK13 cells lysates (not presented).] DNA degradation in MDP-treated RK13 cells incubated with the FMK-linked inhibitors of caspase-3, caspase-8, and caspase-family (C-fam; Z-VAD-FMK) was reduced from MDP-treated culture levels, but was greater than in control cultures (B). Accordingly, the results of the densitometric analysis of DNA bands support significant inhibition of DNA fragmentation by inhibition of initiator caspase-8 (Z-IETD-FMK), effector caspase-3 (Z-DEVD-FMK), and caspase-family (Z-VAD-FMK) inhibitors (41%, 52%, and 75%, respectively) (C). These results suggest that the caspases detected in apoptotic cells plays a role in the acute death of the kidney cell. This assertion is based upon the greater inhibitory effect of the pan-caspase inhibitor (Z-VAD-FMK) than any one of the caspase-specific inhibitors tested. Overall, the results support the early appearance and high levels of initiator caspase-8 and effector caspase-3, and moderate increases in the levels of caspase-1 and initiator caspase-2 and 6 followed closely by activation of mitochondrial caspase-9 and inflammation/stress caspase-10 and -12 in apoptotic RK13 cells. Taken together, the results suggest that multiple caspases were activated in apoptotic rabbit kidney cells, but support key roles for initiator caspase-8 and effector caspase-3 activation. Also, the results suggest that inhibition of the apoptosis may require higher concentrations of caspase-specific inhibitors or more likely the inhibition of multiple caspase.
Concentration-Dependent Inhibition of Apoptosis by Caspase Inhibitors
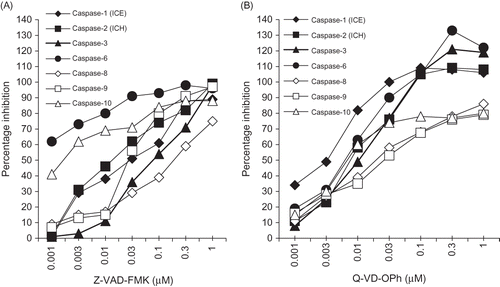
Based upon qualitative differences between caspase inhibitors, dose-dependent inhibition of rabbit caspase-3 activation by each caspase-specific and pan-caspase inhibitor in MDP-induced apoptotic RK13 cells was determined. The 50% inhibitory doses (ID50) for caspase-specific and pan-caspase inhibitors ranged from 0.02 to >10 µM (). The ID50s for the Z-VAD-FMK and Q-VD-OPh (pan-caspase inhibitors) against caspase-3 were 100-fold different (0.2 ± 0.08 and 0.02 ± 0.01 µM, respectively). Concomitantly, the ID50s against caspase-3 activation for caspase-1 (Z-YVAD-FMK), -3, -8, and -10 specific inhibitors ranged between 0.3 and 0.9 µM. The ID50s for caspase -2 (ICH), -4, -9, -12, and -13 specific inhibitors ranged between 0.8 and 4 µM. Inhibition of caspase-3 activation by inhibitors of caspase-5 and -6 was not detected (ID50s >10 µM). Taken together, these results suggest inflammatory caspases (-1, -4, -12, and -13) and initiator/effector caspases (-2, -6, -8, -9, and -10) may activate caspase-3 directly or indirectly via proteolytic activation in apoptotic RK13 cells. The inhibition of caspase-3 by lower concentrations of caspases-1, -8, and -10 specific inhibitors suggest they are likely upstream of caspase-3 activation. However, the lower levels of pan-caspase than caspase-specific inhibitors required to inhibit caspase-3 suggest that multiple caspase likely play a role in the acute MDP-induced rabbit kidney RK13 cell death. [It is important to note that detection and/or inhibition of rabbit caspase-1 and -2 levels were dependent upon the amino acid composition of the substrates used (). That is, caspase-1 activity in RK13 cell lysates was detected using substrates Ac-YEVD-pNA (ICE) and Ac-YEHD-pNA, but not with Ac-YEHD-pNA. Also, more caspase-2 activity was detected with Ac-VDVAD-pNA (ICH) than with Ac-VDQQD-pNA.]
Comparison of Pan-caspase Inhibitors Against MDP-Induced Caspase
Because of the significant difference in the ID50 of the pan-caspase inhibitors and as the effectiveness of the pan-caspase inhibitors would be dependent upon the least effective inhibition of a single caspase in the cascade, it was important to determine if the pan-caspase inhibitors were equally effective in reducing the levels of all MDP-induced rabbit caspases. Thus, the levels of rabbit caspase-1, -2, -3, -6, -8, -9, and -10 activity were compared in MDP-treated RK13 cells incubated in medium containing different concentrations of pan-caspase inhibitors Z-VAD-FMK and Q-VD-OPh for 6 h. Overall, the rabbit caspases were inhibited by each pan-caspase inhibitor in a dose-dependent manner, but some differences were noted (). That is, 1 µM Z-VAD-FMK inhibited all rabbit caspases 85–100%, except for caspase-8 (75%) (A). Z-VAD-FMK effectively inhibited caspase-6 and -10 (A) with ID50s of <0.001 and 0.002 µM, respectively, whereas the ID50 of Z-VAD-FMK for the other caspase tested ranged from 0.01 to 0.1 µM. In comparison, ≥0.1 µM concentration of Q-VD-OPh inhibited caspases-1, -2, -3, and -6 by 90–140% (to or below constitutive levels), but maximal Q-VD-OPh inhibition of caspases-8, -9, and -10 was only 65–85% (B). The ID50s of Q-VD-OPh for MDP-induced caspases ranged from 0.003 to 0.03 µM, with caspase-1 being the most sensitive. The results support dose-dependent differences in the spectrum of rabbit caspases inhibited by Z-VAD-FMK and Q-VD-OPh and suggest less Q-VD-OPh is required to inhibit a broader spectrum of rabbit caspases than Z-VAD-FMK. Notably, Q-VD-OPh inhibited constitutive caspase (especially caspase-1) and higher concentrations of both pan-caspase inhibitors were required to reduce the higher levels of caspase-8 in apoptotic rabbit kidney cells.
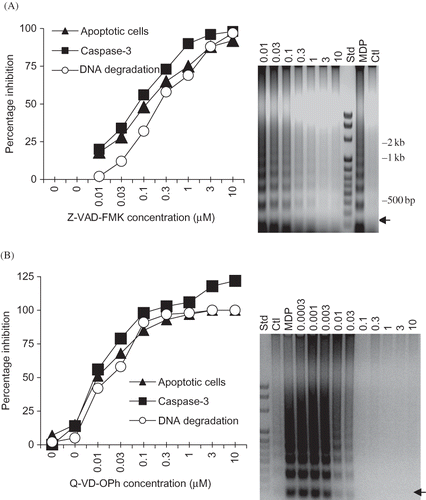
Dose-Dependent Inhibition of Apoptotic Cells, Caspase-3, and DNA Degradation by Pan-caspase Inhibitors
Dose-dependent inhibition of MDP-induced changes in the cell (APOPercentage™ dye uptake), caspase-3 activity, and DNA degradation by Q-VD-OPh and Z-VAD-FMK was compared (). Notably, Z-VAD-FMK concentrations ≥3.0 µM were required to reduce the levels of apoptotic cells, caspase-3 activity, and apoptotic DNA degradation in MDP-treated cells to near control cells (A). Q-VD-OPh concentrations ≥0.1 µM reduced the number of apoptotic cells, caspase-3 activity, and DNA degradation to or below control levels (B). These results suggest that less Q-VD-OPh than Z-VAD-FMK was effective in significantly reducing the number of MDP-induced apoptotic cells, caspase-3 activity, and DNA degradation.
Figure 6. Dose-dependent inhibition of MDP-induced apoptosis (APOPercentageTM-positive apoptotic RK13 cells), caspase-3 activation, and DNA degradation (densitometric analysis of 180 bp DNA bands analyzed; arrows) by (A) Z-VAD-FMK (caspase-family inhibitor), (B) Q-VD-OPh (pan-caspase inhibitor). Each point represents the mean of three experiments. (The gel profiles are representative of the results obtained in three experiments.)
Inhibition of MDP-, PGN-, LPS-, and STP-Induced RK13 Cell Apoptosis by Q-VD-OPh
The next experiments were performed to determine if Q-VD-OPh (1 µM) could inhibit the cell death induced by different muropeptides (MSDP and ADG-MDP), PGN (Streptomyces sp.), STP, and LPS of E. coli and K. pneumoniae (). The inhibition of muropeptides, PGN, and STP-induced caspase-3 activity by Q-VD-OPh (A) paralleled the inhibition of apoptotic DNA degradation (B) and cell death (not shown). These results suggest Q-VD-OPh inhibits muropeptide-, PGN-, LPS-, and STP-induced RK13 cell apoptosis (caspase-3 activation and DNA degradation). Note that LPS-induced caspase-3 activity was inhibited (A), whereas DNA degradation (after 24 h incubation) was less sensitive to Q-VD-OPh (B). These results suggest that Q-VD-OPh reduces the caspase-3 and DNA degradation in RK13 cells incubated with different bacterial cell wall components.
Figure 7. Inhibition of MSDP-, ADG-MDP-, PGN-, STP-, and LPS-induced RK13 cell apoptosis by Q-VD-OPh (1 µM). (A) Mean (±SD) caspase-3 activity in RK13 cells incubated with MSDP, ADG-MDP (1 µg/mL), PGN (250 µg/mL; Streptomyces sp.), STP (1 µg/mL), and LPS [E. coli (Ec) and K. pneumoniae (Kp)] plus (+) and minus (–) Q-VD-OPh for 6 h. The data are from duplicate cultures assayed in triplicate. (B) Apoptotic DNA degradation in RK13 cells (from one of two experiments) incubated with MSDP, ADG-MDP, PGN (Streptomyces sp.), STP, and Ec and Kp LPS plus (+) and minus (–) Q-VD-OPh. (STD; Gene Mark 100 kb DNA ladder.)
![Figure 7. Inhibition of MSDP-, ADG-MDP-, PGN-, STP-, and LPS-induced RK13 cell apoptosis by Q-VD-OPh (1 µM). (A) Mean (±SD) caspase-3 activity in RK13 cells incubated with MSDP, ADG-MDP (1 µg/mL), PGN (250 µg/mL; Streptomyces sp.), STP (1 µg/mL), and LPS [E. coli (Ec) and K. pneumoniae (Kp)] plus (+) and minus (–) Q-VD-OPh for 6 h. The data are from duplicate cultures assayed in triplicate. (B) Apoptotic DNA degradation in RK13 cells (from one of two experiments) incubated with MSDP, ADG-MDP, PGN (Streptomyces sp.), STP, and Ec and Kp LPS plus (+) and minus (–) Q-VD-OPh. (STD; Gene Mark 100 kb DNA ladder.)](/cms/asset/a66ff8d8-9a28-41ea-9bf7-e428cf177753/irnf_a_553304_f0007_b.gif)
Time-Dependent Protective Effect of Pan-caspase Inhibitors
The following experiments were performed to determine (1) the optimal time of pan-caspase inhibitor addition to obtain the maximal inhibitory effect and (2) when pan-caspase inhibitor removal results in loss of the inhibitory effect. In experiments assessing time of pan-caspase inhibitor application, >75% of caspase-3 activity in MDP-treated cultures after 6 h was inhibited when Q-VD-OPh and Z-VAD-FMK were added up to 3 h post 30 min incubation of RK13 cells with MDP (A). Caspase-3 activity was significantly reduced to >40% (p < 0.01) when Q-VD-OPh was added as late as 4 and 5 h post-MDP challenge (i.e., 1 and 2 h prior to termination of the experiment). In comparison, caspase-3 activity was reduced by >20% (p < 0.02) when Z-VAD-FMK was added 4 and 5 h post-MDP challenge. Concomitantly, apoptotic DNA ladders were reduced to control levels in RK13 cell cultures when pan-caspase inhibitors were added during the first 3 h post-MDP (B) (i.e., prior to and during caspase activation) (). The levels of DNA degradation in cultures treated at 4 and 5 h post-MDP were less than in MDP control, consistent with downstream activation of endonucleases associated with late stage cell death at 5–6 h post-MDP. In additional studies not shown, the protective effects of the pan-caspase inhibitors were nullified in the continuous presence of MDP in a time-dependent manner. These results suggest that maximal inhibition of MDP-induced RK13 cell death was achieved earlier with Q-VD-OPh than with a similar inhibitory dose of Z-VAD-FMK and that the inhibitory effect of each pan-caspase inhibitor was less effective if added 3 h after MDP stimulation or in the continued presence of the apoptogenic agent.
Figure 8. Time dependency of pan-caspase inhibition of MDP-induced apoptosis. Effect of addition of 1 µM Q-VD-OPh and 5 µM Z-VAD-FMK at times post-MDP on inhibition of (A) caspase-3 activity and (B) apoptotic DNA degradation. Effect of time of removal of Q-VD-OPh at times post-MDP on (C) inhibition of caspase-3 activity and (D) DNA degradation.
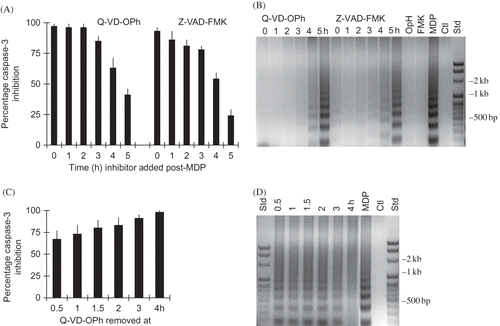
In experiments assessing the effect of time of pan-caspase inhibitor removal, caspase-3 was reduced 67 ± 10% in MDP challenged cultures treated with 1 µM Q-VD-OPh (and 44 ± 12% by 5 µM Z-VAD-FMK, not shown) for 0.5 h (C). The inhibitory effect of Q-VD-OPh on caspase-3 activation increased to maximal levels (>95%) with incubation for 4 h before removal (C). In contrast, apoptotic DNA ladders were reduced (>50%) from the MDP-treated cells treated with 1 µM Q-VD-OPh (and 5 µM Z-VAD-FMK, not shown) for 0.5–3 h, but were not detected in cultures treated for 4 h before pan-caspase inhibitor removal (D). These results are consistent with the suggestion that effective inhibition of apoptosis is achieved when an intracellular inhibitory concentration of each pan-caspase inhibitor was present at the time of caspase activation (i.e., consistent with irreversible binding of inhibitor substrate to activated caspases).
DISCUSSION
The acute induction of rabbit kidney cell apoptosis by very low concentrations of muropeptides containing l-alanyl-d-isoglutamine stereo-isomers and high concentrations of PGN and LPS from different bacteria is consistent with the possibility that the interaction of bacterial cell wall components with kidney cells induces the acute apoptosis observed in septic animals with ARF.Citation2,Citation3 Moreover, multiple initiator (caspase-2, -8, -9, and -10), effector (caspase-3 and -6/7), and inflammatory (caspase-1) caspases were activated in the apoptotic rabbit kidney cells. The activation of multiple caspases suggests that death receptor, mitochondrial, and ER/stress-mediated apoptotic pathways may be activated in rabbit kidney cells by MDP, PGN, and LPS. The inhibition of rabbit kidney cell death by broad-spectrum caspase inhibitors, rather than individual caspase inhibitors, supports the likelihood that multiple caspases play a pathogenic role in the RK13 cell death. Further, the role of multiple caspases is supported by the protective effect of pan-caspase inhibitors against bacterial cell wall-induced RK13 cell death and is consistent with the reported beneficial effect of pan-caspase inhibition against ARF.Citation3
A key question in the pathogenesis of ARF in septic animal models and man is the nature of the renal cell apoptotic stimulus. Our results suggest that apoptotic rabbit kidney cell death induced by bacterial cell wall components proceeds via activation of extrinsic and intrinsic pathways. The similarity in caspase cascades suggests convergence or triggering of a common signal pathway(s) by synthetic MDPs and high concentrations of PGN and LPS from different bacteria that is upstream of initiator caspase activation. That is, the activation of caspase-8, -3, and -10 in rabbit kidney cells suggests FADD activation via death receptor activation by bacterial cell wall components.Citation26 This result is consistent with our previous studies showing FADD-mediated rabbit kidney cell apoptosis is initiated by Ca2+-dependent binding of MDP to cell surface CRT of the GGT-positive RK13 cells.Citation17,Citation19 The activation of caspase-2 and -9 suggests activation of ER/stress and mitochondrial apoptotic pathways possibly via perturbation of CRT-dependent functions, such as the regulation of the intracellular Ca2+ or modulation of the association of mitochondria with the smooth ER.Citation8,Citation9,Citation27 In this regard, it is of interest to note that tubular cell Ca2+ concentration and content rise following acute renal injury induced by LPS, ischemic, and toxic insults and that the proximal tubule appears to be a major site of injury in ARF.Citation28 Taken together, the results suggest that the caspase-mediated rabbit kidney cell apoptosis induced by bacterial cell wall components likely involves stimuli-specific recruitment of different apoptotic pathways, but may be due to redundancy and/or convergence of signal pathways induced by MDP, PGN, and LPS,Citation29 stimulation via the nucleotide oligomerization domain,Citation4 caspase-2/8 cleavage of mitochondrial Bcl/Bid with cytochrome c release,Citation30,Citation31 and caspase cross-talk.Citation32 Additional studies are required to fully characterize the role of CRT/Ca2+ in the stimulation of different apoptotic pathways in renal cells and tissue.
Pan-caspase inhibitors were qualitatively and quantitatively more effective than the caspase-specific inhibitors at inhibiting cell death, caspase-3 activation, and DNA degradation. The pan-caspase inhibitors were effective inhibitors of activated rabbit caspases when added during the stimulus-to-response lag time, but significant differences were observed between the pan-caspase inhibitors. That is, higher concentrations of Z-VAD-FMK than Q-VD-OPh were required to significantly reduce MDP-induced cell death, possibly due to differences in cell permeability.Citation33 Further, ≥30-fold Z-VAD-FMK than Q-VD-OPh was required to reduce DNA degradation to near control levels. Interestingly, rabbit caspase-6 and -10 were the most sensitive, whereas caspase-8 was less sensitive than caspase-3 to Z-VAD-FMK inhibition. Most rabbit caspases were sensitive to Q-VD-OPh (ID50s ranged from 0.003 to 0.01 µM) with caspase-8 and -9 being the least sensitive. Concomitantly, Q-VD-OPh concentrations of >0.1 µM reduced constitutive levels of rabbit caspase-1, -2, -3, and -6 activities. It should be noted that the long-term inhibition of constitutively expressed caspases important to cell differentiation could have detrimental or toxic effects. Pan-caspase inhibitors significantly reduced caspase-3 levels in LPS-treated RK13 cells, but did not inhibit apoptotic DNA degradation/cell death. The failure to inhibit MDP/PGN-induced DNA degradation by Z-VAD-FMK and LPS-induced DNA degradation by both pan-caspase suggests that caspase-independent cell death pathways may be activated. However, autophagosomes and toxic effectsCitation33 were not detected in MDP-, PGN-, or LPS-treated RK13 cells incubated in media containing FMK, difluorophenoxy-methyl ketone (OPh), or pan-caspase inhibitors. The efficiency of the pan-caspase inhibitors against apoptosis induced by bacterial cell components, especially MDP, was increased by the continuous presence of the pan-caspase inhibitor and the removal of the bacterial component from the culture. Thus, our results suggest that the in vivo efficiency of pan-caspase inhibitors likely depends on the dose, time of application, and rate/efficiency of renal clearance of the inhibitor and bacterial components.Citation34
The idea that the septic inflammatory response is initiated by the apoptotic cells is based upon the observation that administration of pan-caspase inhibitors reduces inflammation and apoptosis in septic patients and animals.Citation1–4 The detection of increased caspase-1 activity and immunoreactive IL-1β in apoptotic rabbit kidney cells incubated with bacterial cell wall components suggests that the apoptotic renal cells release inflammatory cytokines. This result is consistent with LPS-induced caspase-1 in ARFCitation3 and MDP induction of caspase-1 in the mouse eye,Citation35 but the MDP-induced ocular inflammation in the mouse eye was independent of IL-1β signaling.Citation35 However, caspase-1 appears to play a role in Pseudomonas-induced keratitisCitation36 and Shigella-induced inflammation.Citation37 Additional studies are required to identify the inflammatory caspases and cytokines induced by bacteria in kidney cells and determine their role in apoptotic-cell-induced inflammation.
In summary, the results suggest that MDP, PGN, and LPS stimulate multiple caspases and cell death in rabbit renal tubule-like cells and support a pathogenic role for bacterial cell wall components in acute renal tubule apoptosis. The inhibition of rabbit kidney cell apoptosis and ARFCitation3 by pan-caspase inhibitors suggests that multiple caspases mediate bacterial cell wall-induced kidney cell apoptosis. The induction of caspase-mediated apoptotic cell death by bacteria/bacterial components in lymphocytes,Citation38 vascular endothelial,Citation39 lung (Staphylococcus-induced) and intestinal epithelial (H. pylori-induced),Citation40,Citation41 meningeal,Citation42 uveal ciliary body epithelial,Citation43 and renal tubule cells supports the concept that bacterial cell wall components induce apoptosis in multiple tissues/organs.
Acknowledgments
The authors thank Christopher Duggan for excellent technical assistance. This research was supported by the Faculty Improvement Fund and an unrestricted grant from Allergan to support education and research by LSUHSC-Shreveport Ophthalmology Faculty and Residents.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kang YH, Falk MC, Bentley TB, Lee CH. Distribution and role of lipopolysaccharide in the pathogenesis of acute renal proximal tubule injury. Shock. 1995;4:441–449.
- Jo SK, Cha DR, Cho WY, Inflammatory cytokines and lipopolysaccharide induce Fas-mediated apoptosis in renal tubular cells. Nephron. 2002;91:406–415.
- Guo R, Wang Y, Minto AW, Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol. 2004;15:3093–3102.
- Cartwright N, Murch O, McMaster SK, Selective NOD1 agonists cause shock and organ injury/dysfunction in vivo. Am J Respir Crit Care Med. 2007;174:595–603.
- Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol. 2004;172:2629–2635.
- Chang KC, Unsinger J, Davis CG, Multiple triggers of cell death in sepsis: Death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719.
- Wan L, Bagshaw SM, Langenberg C, Pathophysiology of septic acute kidney injury: What do we really know? Crit Care Med. 2008;36(Suppl. 4):S198–S203.
- Nakamura K, Bossy-Wetzel E, Burns K, Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol. 2000;150:731–740.
- Slee EA, Harte MT, Kluck RM, Ordering the cytochrome c-initiated caspase cascade: Hierarchial activation of caspases-2,-3,-6,-7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292.
- Nhan TQ, Liles WC, Schwartz SM. Physiological functions of caspases beyond cell death. Am J Pathol. 2006;169:729–737.
- Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281:1312–1316.
- Lavrik IN, Golks A, Krammer PH. Caspases: Pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–2672.
- Wesche-Soldato DE, Swan RZ, Chung C-S, Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets. 2007;8:493–500.
- Wan L, Bellomo R, Di Giantomasso D, Ronno C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care. 2003;9:496–502.
- Waters RV, Terrel TG, Jones GH. Uveitis induction in rabbit by muramyl dipeptides. Infect Immun. 1986;51:816–825.
- Yokota M, Kambayashi J, Tahara H, Renal insufficiency induced by locally administered endotoxin in rabbits. Methods Find Exp Clin Pharmacol. 1990;12:487–491.
- Langford MP, Chen D, Welbourne TC, Redens TB, Ganley JP. Stereo-isomer specific induction of renal cell apoptosis by synthetic muramyl dipeptide (N-acetylmuramyl-l-alanyl-d-isoglutamine). Molec Cell Biochem. 2002;236:63–73.
- Beale AJ, Christofinis GC, Furminger IGS. Rabbit cells susceptible to rubella virus. Lancet. 1963;2:640–641.
- Chen D, Duggan C, Redens TB, Kooragayala LM, Texada DE, Langford MP. Calreticulin is a binding protein for muramyl dipeptide and peptidoglycan in RK13 cells. Biochemistry. 2004;43:11796–11801.
- Chen D, Texada DE, Duggan C, Surface calreticulin mediates muramyl dipeptide induced apoptosis in RK13 cells. J Biol Chem. 2005;280:22425–22436.
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666.
- Choi KB, Wong F, Harlan JM, Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem. 1998;273:20185–20188.
- Andersson M, Sjostrand J, Petersen A, Honarvar AKS, Karlsson J-O. Caspase and proteosome activity during staurosporin-induced apoptosis in lens epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:2623–2632.
- Carretti DP, Kozlosky CJ, Mosley B, Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100.
- Chen D, Texada DA, Duggan C, Deng Y, Langford MP. Caspase-3 and -7 mediate apoptosis of human Chang's conjunctival cells induced by enterovirus 70. Virology. 2006;347:307–322.
- Askenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:305–308.
- Wang H-J, Guay G, Pogan L, Sauve R, Nabi IR. Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum. J Cell Biol. 2000;150:1489–1497.
- Sanz AB, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol. 2008;19:1634–1642.
- Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem J. 2007;404:179–190.
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;4:491–501.
- Upton J-P, Austgen K, Nishino M, Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:3943–3951.
- Roy S, Nicholson DW. Cross-talk in cell death signaling. J Exp Med. 2000;192:F21–F25.
- Caserta TM, Smith AN, Gultice AD, Reedy A, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352.
- Zikos D, Krueger J, Peterson DR. Renal metabolism of muramyl dipeptide. Contrib Nephrol. 1988;68:19–25.
- Rosenzweig HL, Martin TM, Planck SR, Activation of NOD2 in vivo induces IL-1beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J Leukoc Biol. 2008;84:529–536.
- Thakur A, Barrett RP, Hobden JA, Hazlett LD. Caspase-1 inhibitor reduces severity of Pseudomonas aeruginosa keratitis in mice. Invest Ophthalmol Vis Sci. 2004;45:3177–3184.
- Sansonetti PJ, Phalipon A, Arondel J, Caspase-1 activation of IL-1β and Il-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590.
- Hotchkiss RS, Tinsley KW, Swanson PE, Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546.
- Sylte MJ, Leite FP, Kuckleburg CJ, Inzana TJ, Czuprynski CJ. Caspase activation during Hemophilus somnus lipooligosaccharide-mediated apoptosis of bovine endothelial cells. Microb Pathog. 2003;35:285–291.
- Schmeck B, Gross R, N'Guessan PD, Streptococcus pneumoniae-induced caspase 6-dependent apoptosis in lung epithelium. Infect Immun. 2004;72:4940–4947.
- Ashktorab H, Neapolitano M, Bomma C, In vivo and in vitro activation of caspase-8 and -3 associated with Helicobacter pylori infection. Microbes Infect. 2002;4:713–722.
- Braun JS, Prass K, Dirnagl U, Meisel A, Meisel C. Protection from brain damage and bacterial infection in murine stroke by the novel caspase-inhibitor Q-VD-OPH. Exp Neurol. 2007;206:183–191.
- Langford MP, Chen D, Gosslee J, Intracameral toxicity of bacterial components muramyl dipeptide and staurosporine; ciliary cyst formation, epithelial cell apoptosis and necrosis. Cutan Ocular Toxicol. 2006;25:85–101.