Abstract
Background: Amiodarone is a class III antiarrhythmic agent that is widely used in the treatment of a variety of arrhythmias. Several different systemic side effects are reported after use of this medication. In this article, we report a case that had developed syndrome of inappropriate antidiuretic hormone (SIADH) after starting treatment with this agent. Case report: The patient is a 66-year-old male with past medical history of hypertension, hyperlipidemia, coronary artery disease, and class III New York Heart Association congestive heart failure who presented with monomorphic nonsustained ventricular tachycardia. A loading dose of amiodarone followed by maintenance dose was started. Baseline serum sodium of 138 mmol/L on admission decreased to 119 mmol/L by day 7, and a diagnosis of SIADH was made. The patient was not taking any other medication known to cause SIADH, nor had any such comorbidity to explain it. Serum sodium increased to 133 and 138 mmol/L, respectively, after 16 and 33 days from discontinuation of amiodarone. Conclusion: SIADH is a rare but serious side effect of amiodarone and practicing physicians should be aware of this complication, particularly after loading dose of the medication.
INTRODUCTION
Syndrome of inappropriate antidiuretic hormone (SIADH) is a partial impairment of water excretion due to the inability to suppress excretion of ADH.Citation1 The severity of hyponatremia is determined by the amount of fluid ingested and the degree of impairment of water excretion.
Amiodarone is a class III antiarrhythmic agent that is used in the treatment of a variety of arrhythmias. It also has many different systemic side effects and complications.Citation2,Citation3 In this report, we present a case of SIADH induced by amiodarone.
CASE REPORT
The patient is a 66-year-old man with a past medical history of hypertension, hyperlipidemia, coronary artery disease, status post three-vessel coronary artery bypass in 2005, and class III compensated New York Heart Association congestive heart failure (CHF) with ejection fraction of 15% who was admitted on 21 September 2010 for elective placement of biventricular pacemaker and automated implantable cardioverter-defibrillator. He denied any chest pain or dyspnea on admission and was in usual state of health without any decompensation of CHF. He had occasional dizziness associated with dyspnea on exertion as outpatient without any other symptoms. His home medications were lisinopril 40 mg orally once a day, carvedilol 12.5 mg orally twice a day, simvastatin 10 mg orally once every night, aspirin 81 mg orally once a day, and furosemide 40 mg orally once every other day. He was not using any medication at home known to induce SIADH.
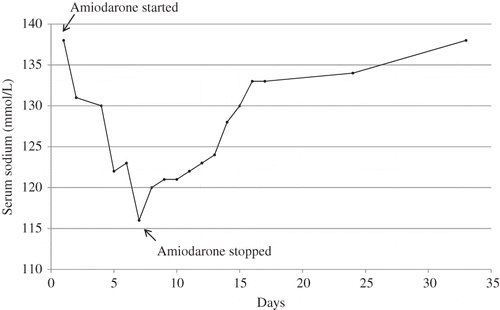
On admission, his blood pressure was 141/101 mmHg, pulse rate 66 per minute, respiratory rate 14 per minute, temperature 99˚F, and O2 saturation 96% in room air. He was alert, awake, and in no cardiorespiratory distress. His heart and lung examinations were unremarkable. Abdomen was soft, without tenderness, and with normal bowel sounds. There was no significant lower extremity edema. Laboratory findings on admission included serum sodium 138 mmol/L, potassium 4.7 mmol/L, chloride 104 mmol/L, HCO3 27 mmol/L, glucose 120 mg/dL, blood urea nitrogen (BUN) 17 mg/dL, and creatinine 0.9 mg/dL. On the first day of admission, he developed a nonsustained monomorphic ventricular tachycardia (VT) without any evidence of clinical exacerbation of CHF. Amiodarone 150 mg intravenous over 10 min was given, which was followed by 900 mg drip over 24 h, followed by 400 mg orally three times a day intended to be given for the next 1 week. Starting from the next day of dispensing amiodarone, the patient's serum sodium gradually declined to 116 mmol/L on day 7 (). At this point urine osmolarity was 644 mOsm, urine sodium 90 mmol/L, serum osmolarity 262 mOsm, uric acid 4.3 mg/dL, cortisol 7 µg/dL, free T3 2.3 pg/mL, free T4 0.88 ng/dL, and thyroid-stimulating hormone (TSH) 5.66 µIU/mL. The patient remained clinically euvolemic at all times during the hospital stay. Chest X-ray was clear and did not show any evidence for pulmonary edema or mass suggestive of malignancy. Based on laboratory and clinical findings, a diagnosis of SIADH was made. He was not prescribed any medication during the hospital stay known to cause SIADH. As no other explanation for SIADH was found, amiodarone was discontinued on day 7 as shown in . The trend of decreasing serum sodium was reversed the next day and sodium continued to increase until day 16 when the patient was discharged home in stable condition. On day 33, repeat serum sodium as outpatient revealed normal sodium level of 138 mmol/L.
DISCUSSION
In our patient, SIADH developed rapidly after initiating amiodarone. Serum sodium started to increase immediately after discontinuation of the medication. The patient was not given any other medication known to induce SIADH. He was euvolemic at all times, with stable and compensated heart function and without exacerbation of his underlying CHF. There was not any evidence for adrenal insufficiency or hypothyroidism based on the medical work-ups. As there has not been any other identifiable cause for SIADH, the temporal decline of serum sodium with loading dose of amiodarone and rise after its discontinuation suggests amiodarone as the etiologic agent for SIADH in this patient.
The first report of hyponatremia during treatment with amiodarone was presented by Odeh in 1999.Citation4 In a 62-year-old female with a history of hypertension and paroxysmal atrial fibrillation who had been taking amiodarone 300 mg per day and was admitted for a complaint of palpitations, low serum sodium of 120 mmol/L was observed on admission. After diagnosing SIADH and discontinuation of amiodarone, serum sodium increased to 133 and 143 mmol/L in 5 and 14 days, respectively. Ikegami et al.Citation5 reported two cases of SIADH induced by amiodarone. A 60-year-old man with a history of idiopathic dilated cardiomyopathy presented with recurrent sustained VT. Seven days after start of amiodarone 800 mg per day, his serum sodium level decreased to 119 mmol/L. In this case after reduction of amiodarone to 100 mg per day and a more stringent restriction of fluid intake, serum sodium normalized within 1 month after dose reduction. In a second case reported by Ikegami, an 87-year-old man was admitted with recurrent sustained VT associated with myocardial infarction. Fifteen days after start of amiodarone, serum sodium was 121 mmol/L. In this case a low dose of amiodarone (100 mg daily) was continued along with moderate fluid restriction and serum sodium increased to 133 mmol/L within 2 weeks. Patel et al.Citation6 presented a 67-year-old male with a history of chronic obstructive pulmonary disease, coronary artery disease, and CHF who presented to emergency with abdominal pain. A serum sodium of 117 mmol/L was observed. Retrospective review of earlier events showed decline of serum sodium from 134 mmol/L 3 months earlier to 126 mmol/L within a few days after starting amiodarone, as outpatient. After discontinuation of the drug, serum sodium was 129 and 136 mmol/L after 3 and 30 days, respectively. Aslam et al.Citation7 reported a 72-year-old man with a history of nonischemic dilated cardiomyopathy, paroxysmal atrial fibrillation, and sustained monomorphic VT who was admitted for amiodarone loading for VT refractory to other agents. Four days after loading with 2 g per day of amiodarone for 5 days, serum sodium was 117 mmol/L. After reduction of the dose to 200 mg daily, serum sodium stabilized after 14 days to 130–135 mmol/L. A second decline in serum sodium was observed after reloading with amiodarone in an attempt to manage a stable sustained monomorphic VT, 3 weeks later, which was improved after resuming the maintenance dose of 200 mg per day. Shavit and ShererCitation8 presented an 85-year-old male with a history of hypertension, coronary artery disease, and atrial fibrillation who was admitted with chest pain, abdominal pain, and weakness. One month before this admission, he underwent percutaneous transluminal coronary angioplasty and was started on treatment with amiodarone. On admission, his serum sodium was 122 mmol/L. After discontinuation of amiodarone, his serum sodium was normalized in a few days and remained stable in a 3-month follow-up visit. Recently, Paydas et al.Citation9 have reported a 58-year-old male with a history of hypertension and CHF who presented with abdominal pain and nausea. Five months earlier he was started on amiodarone when diagnosis of CHF was made, while having normal baseline serum sodium. On admission, serum sodium was 107 mmol/L and SIADH was diagnosed. After discontinuation of amiodarone, serum sodium started to increase within 3 days and normalized within 1 month. In none of the above reports with diagnosis of SIADH, other medications known to cause SIADH could be found. Similarly, no other clinical comorbidities that could explain SIADH were identified.
The exact mechanism of induction of SIADH with amiodarone is not defined. Its mechanistic effects on the central nervous system as well as on renal tubules and collecting ducts are yet to be explained. It is unclear if there is any genetic linkage that may explain susceptibility for induction of SIADH following administration of amiodarone. Increase of serum sodium after decreasing the dose or switching the loading dose to maintenance dose suggests that the underlying mechanism may be somehow dose dependent. It is also unclear if any of the underlying comorbidities act as predisposing factor or increase the risk of developing amiodarone-induced SIADH. Although this complication is uncommon, it can be quite severe. In summary, physicians should be aware of potentially life-threatening hyponatremia induced by amiodarone.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Rose B, Post T, eds. Clinical Physiology of Acid-Base and Electrolyte Disorders. 5th ed., New York: McGraw-Hill; 2001.
- Hilleman D, Miller MA, Parker R, Doering P, Pieper JA. Optimal management of amiodarone therapy: Efficacy and side effects. Pharmacotherapy. 1998;18:138S–145S.
- Wilson JS, Podrid PJ. Side effects from amiodarone. Am Heart J. 1991;121:158–171.
- Odeh M, Schiff E, Oliven A. Hyponatremia during therapy with amiodarone. Arch Intern Med. 1999;159:2599–2600.
- Ikegami H, Shiga T, Tsushima T, Nirei T, Kasanuki H. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) induced by amiodarone: A report on two cases. J Cardiovasc Pharmacol Ther. 2002;7:25–28.
- Patel GP, Kasiar JB. Syndrome of inappropriate antidiuretic hormone-induced hyponatremia associated with amiodarone. Pharmacotherapy. 2002;22:649–651.
- Aslam MK, Gnaim C, Kutnick J, Kowal RC, McGuire DK. Syndrome of inappropriate antidiuretic hormone secretion induced by amiodarone therapy. Pacing Clin Electrophysiol. 2004;27:831–832.
- Shavit E, Sherer Y. Hyponatremia induced by amiodarone therapy. Isr Med Assoc J. 2007;9:564–565.
- Paydas S, Araz F, Balal M. SIADH induced by amiodarone in a patient with heart failure. Int J Clin Pract. 2008;62:337.