Abstract
Aim: In the past years, aldosterone has been identified as an important mediator of renal injury. In this study, we evaluated the influence of C-344T polymorphism of aldosterone synthase gene, associated with serum aldosterone levels and the development of arterial hypertension, on IgA nephropathy (IgAN). Methods: We studied n = 143 patients with biopsy-proven IgAN followed up for 7.1 ± 6.2 years. Patients were classified according to the slope of reciprocal serum creatinine into group A (slow progressors, n = 93) and group B (fast progressors, n = 50). One hundred healthy volunteers were analyzed as controls. The biopsies of n = 79 patients were reviewed and analyzed by the same pathologist. Aldosterone synthase gene C-344T polymorphism was determined by polymerase chain reaction amplification. Results: The genotype distribution was similar in patients and control subjects [not significant (ns)]. Age, initial renal function, proteinuria, and blood pressure did not differ significantly between patients with different genotypes (ns). The percentage of sclerosed glomeruli tended to be higher among patients carrying the CC/CT genotypes (29.4 ± 26.5% vs. 21.7 ± 25.2% in TT genotype; ns). C-344T polymorphism was associated with the progression of IgAN as shown by the different genotype frequencies in group Α (slow progressors, CC/CT: 60.2%, TT: 39.8%) and group B (fast progressors, CC/CT: 78.0%, TT: 22:0%; p = 0.032). Conclusion: Our results indicate that aldosterone synthase gene C-344T polymorphism is a risk factor for accelerated progression in Caucasian patients with IgAN.
INTRODUCTION
IgA nephropathy (IgAN) is the most common glomerulonephritis and a considerable cause of end-stage renal disease. Characterized by mesangial deposition of immunoglobulin A, matrix expansion, and cellular proliferation, IgAN has a very variable clinical course ranging from stable renal function over decades to terminal renal failure within few years. Clinical and histological predictors including impairment of renal function, severe proteinuria, arterial hypertension at presentation, and interstitial fibrosis have been associated with an unfavorable outcome, but clear-cut criteria for an individual prognosis and targeted therapy have not yet been established.
The renin–angiotensin–aldosterone system (RAAS) is believed to play a central role in glomerular diseases, not only through the regulation of arterial blood pressure and local tissue hemodynamics, but also through the actions of its end products on the function and architecture of the cellular structures of the kidney. Trials showing the beneficial effects of angiotensin-converting enzyme (ACE) inhibitors in IgAN emphasized the pivotal role of the RAAS in determining the rate of progression.Citation1 Previously described associations of genes encoding components of the RAAS with the progression of IgAN underline this fact.Citation2
Although angiotensin-II is considered as the major mediator of the RAAS, recent evidence also implicates aldosterone as an important factor in progressive renal disease. Originally, aldosterone was thought to be produced uniquely in the adrenal cortex and to act exclusively on epithelia to promote sodium retention and potassium excretion. However, it is now well established that aldosterone acts also on nonepithelial tissues, such as cardiac and vascular endothelial cells, having nonclassical nonhemodynamic actions.Citation3 Aldosterone contributes to inflammatory and fibrotic effects that were previously attributed solely to angiotensin-II and promotes nephrosclerosis in a fashion that cannot be directly attributed to hemodynamic effects.Citation3 Furthermore, aldosterone synthase required for aldosterone biosynthesis are expressed in the normal glomerulus, which may be consistent with local aldosterone production acting in an autocrine fashion.Citation4
In view of the increasing importance of aldosterone as a mediator of renal injury, in this study we evaluated the influence of the C-344T polymorphism of aldosterone synthase gene, associated with serum aldosterone levels and the development of arterial hypertensionCitation5 on the clinical course of IgAN. Our aim was to identify genetically determined subgroups of patients with IgAN who had a more rapid decline in renal function.
PATIENTS AND METHODS
Patients and Controls
We studied n = 143 Caucasian patients with biopsy-proven IgAN. The mean follow-up period was 7.1 ± 6.2 years and included monitoring of serum creatinine, proteinuria, blood pressure, and antihypertensive medication. Arterial hypertension was defined by the presence of blood pressure values over 130 mmHg systolic and/or 80 mmHg diastolic or the need for antihypertensive treatment. A mean of 15.7 ± 13 data points was retrieved. Only patients followed up for at least 1 year were included. The slope of reciprocal serum creatinine (1/Cr) against time was used to evaluate the progression of the disease. Patients were classified accordingly into group A (≥–0.1 dL × mg−1 × year−1, slow progressors, n = 93) and group B (<–0.1 dL × mg−1 × year−1, fast progressors, n = 50). None of the patients were treated with an aldosterone receptor antagonist. The biopsies of n = 79 patients were reviewed and analyzed by the same pathologist, who was unaware of the phenotype (clinical outcome) and genotype of the patients. In addition to establishing the diagnosis of IgAN, the percentage of global sclerosed glomeruli, the percentage of tubulointerstitial area showing fibrosis, and the degree of arteriosclerosis were estimated. One hundred volunteers served as a control group. Their mean age was 40 ± 16 years. This study was approved by the local ethics committee.
Determination of Aldosterone Synthase (CYP11B2) Gene C-344T Polymorphism
Genomic DNA was extracted from peripheral leukocytes from whole blood samples using the QIAmp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). To detect the aldosterone synthase (CYP11B2) gene C-344T polymorphism, a polymerase chain reaction (PCR) amplification followed by restriction digestion with the endonuclease HaeIII was carried out. The primers used were sense: 5′ CAG GAG GAG ACC CCA TGT GAC 3′, antisense: 5′ CCT CCA CCC TGT TCA GCC C 3′ (MWG Biotech AG, Ebersberg, Germany). There was an initial denaturation at 95°C for 15 min followed by 32 cycles consisting of denaturation at 95°C for 40 s, annealing at 60°C for 40 s, and extension at 72°C for 40 s, followed by a final extension step at 72°C for 5 min. The amplified products were incubated for 3 h with the endonuclease HaeIII (New England Biolabs, Frankfurt am Main, Germany). In the -344T allele, the initial 538 bp long PCR product was divided into three smaller fragments (273, 138, and 128 bp). In the presence of the -344C allele, the 273 bp band was further divided in two smaller pieces (202 and 71 bp).
Statistical Analysis
Data were expressed as percentages or means ± standard deviation. Pearson's chi-square was used for categorical data. Continuous variables were tested in each group for normal distribution using the Kolmogorov–Smirnov test for one variable. Differences between two groups were tested with the Student's t-test or the nonparametric Mann–Whitney U-test. For correlations between continuous variables, Pearson's or the nonparametric Spearman's tests were used. The individual rate of progression of renal insufficiency was calculated as the slope of reciprocal serum creatinine versus time plot (linear regression). Multivariate analysis was performed using multivariate logistic regression. All tests were two-sided and statistical significance was defined as p < 0.05.
RESULTS
Patients’ Characteristics
Our study population consisted of n = 143 Caucasian patients (105 males and 38 females). Their mean age at diagnosis was 37.5 ± 14.0 years. The mean serum creatinine was 2.1 ± 1.4 mg/dL and the mean proteinuria 3.8 ± 2.9 g/24 h. The slope of reciprocal serum creatinine against time was used to evaluate the progression of the disease. Its mean value was −0.140 ± 0.403 dL × mg−1 × year−1 (represents a rise of serum creatinine from 1.0 to 2.0 mg/dL in a period of 3.5 years). Patients were classified accordingly into group A (≥–0.1 dL × mg−1 × year−1, slow progressors, n = 93) and group B (<–0.1 dL × mg−1 × year−1, fast progressors, n = 50). As far as nongenetic factors were concerned, patients in the fast progressing group B had worse renal function already at the time of renal biopsy (p < 0.05), higher proteinuria (p < 0.001), and higher blood pressure values (p < 0.05). Treatment with ACE inhibitors had a protective effect: 28.2% of the patients on ACE inhibition compared with 51.1% of the untreated patients belonged to the fast progressing group B (χ2: 6.82, OR: 2.65, 95% CI: 1.26–5.57, p < 0.01).
Histological Features, Initial Renal Function, and Progression
The initial renal function correlated to the degree of glomerular sclerosis (r = 0.403, p < 0.001), tubulointerstitial fibrosis (r = 0.542, p < 0.001), and arteriosclerosis (r = 0.488, p < 0.001). The percentage of sclerosed glomeruli tended to be higher in the fast progressing group B [34.0 ± 32.7% vs. 24.2 ± 23.8% in group A, not significant (ns)].
Aldosterone Synthase gene C-344T Genotype and Incidence of IgAN
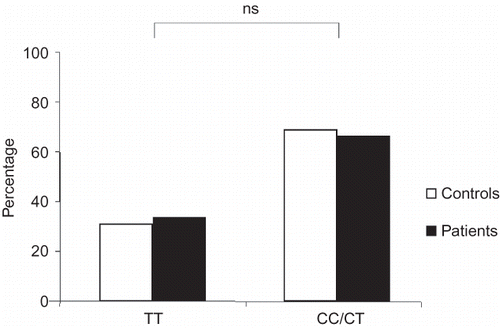
The genotype frequencies of the C-344T polymorphism were similar in patients (CC/CT: 66.4%, TT: 33.6%) and control subjects (CC/CT: 69%, TT: 31%, ns, ). The observed genotype frequencies corresponded in both groups to the expected values according to the Hardy–Weinberg equilibrium.
Aldosterone Synthase Gene C-344T Genotype and Parameters at Renal Biopsy
There were no relevant differences at the time of renal biopsy in terms of age, renal function, proteinuria, and blood pressure between patients with different C-344T genotypes (ns, ). Furthermore, no significant difference in the number of patients treated with ACE inhibitors or angiotensin-II receptor antagonists was observed (ns, ). The percentage of sclerosed glomeruli tended to be higher among patients carrying the CC/CT genotypes (29.4 ± 26.5% vs. 21.7 ± 25.2% in TT genotype, ns).
Table 1. Clinical and laboratory parameters at the time of renal biopsy (mean ± SD) in n = 143 patients with IgA nephropathy according to the aldosterone synthase gene C-344T genotype
Aldosterone Synthase Gene C-344T Genotype and Progression of IgAN
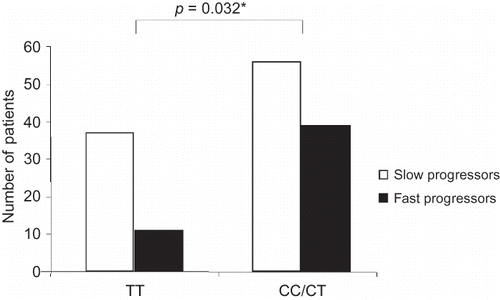
The C-344T polymorphism was associated with the progression of IgAN: a significant difference in the genotype distribution between the two groups was found, with the CC/CT genotypes (carriage of the C-allele) being more frequent among fast progressors (, χ2: 4.61, OR: 2.34, 95% CI: 1.07–5.15, p = 0.032). The slope of reciprocal serum creatinine tended to be higher in patients with the CC/CT genotypes (–0.168 ± 0.486) compared with the TT genotype (–0.085 ± 0.121 dL × mg−1 × year−1, p = 0.163). In the multivariate analysis, among the other clinical factors only proteinuria (p = 0.001), ACE inhibition (p = 0.026), and aldosterone synthase gene polymorphism (p = 0.030) remained independent predictors of progression.
DISCUSSION
In the past years, aldosterone has been identified as an important contributor to the progression of kidney disease. In this study, we evaluated the impact of the C-344T polymorphism of aldosterone synthase gene on IgAN.
The CYP11B2 gene encodes for aldosterone synthase, which catalyzes the terminal steps of aldosterone biosynthesis. The C-344T polymorphism is located in the promoter region of the CYP11B2 gene in a putative binding site for the steroidogenic transcription factor SF-1.Citation6 The -344C allele binds SF-1 about four times more effectively than the T-allele.Citation6 The presence of the -344C allele, which was associated with a worse outcome in our study population, was linked to elevated levels of serum aldosterone in a cohort of 216 patients of European origin (TT genotype: 90 ± 8, CT: 110 ± 6 and CC: 129 ± 10 pg/mL).Citation7 In another cohort of 562 Caucasian patients, higher serum aldosterone levels were also observed in the CC genotype (TT genotype: 121 ± 7, CT: 125 ± 5, CC: 131 ± 7 pmol/L), but the difference was not statistically significant.Citation8 A smaller study (n = 114) however reported an opposite association, that is, higher serum aldosterone levels in the TT genotype (TT: 164 ± 100, CT: 194 ± 108, CC: 130 ± 62, notice the high standard deviation and the fact that the heterozygotes have the highest levels).Citation9 No data exist regarding the impact of aldosterone synthase gene C-344T polymorphism on aldosterone tissue levels. Furthermore, an increased susceptibility for the development of arterial hypertension was reported in the -344C allele by some investigators,Citation10,Citation11 but no impactCitation7,Citation8 or even the oppositeCitation5 was reported by other authors. Several reasons could account for this inconsistency: These studies are performed in populations with different ethnic backgrounds. It is now well understood that results of genetic studies cannot be transferred from one race to another without confirmation, because the expression of certain genetic variations might be differently regulated in different races. A limitation of most of these studies is sample size, given the fact that they are performed in rather small cohorts of patients, and they might be underpowered to detect an impact on a disease of such a multifactorial origin, for example, arterial hypertension. Furthermore, they are retrospective. This means that medication and particular ACE inhibitors and aldosterone receptor antagonists are not uniformly used or avoided. Moreover, sodium intake, which is a major regulator of aldosterone activity and has major interindividual and intercultural variations, was not standardized in these studies.
In this study, we investigated the relationship of aldosterone synthase gene C-344T polymorphism with the incidence and clinical outcomes in patients with IgAN. No significant difference was observed in the allele frequencies of patients and controls. Thus, the examined polymorphism gave no indication of a predisposition for the development of IgAN. Although not statistically significant, the percentage of sclerosed glomeruli tended to be higher in patients carrying the CC/CT genotypes (C-allele carriers). A significant association of the aldosterone synthase gene polymorphism with the progression of IgAN was detected: the CC/CT genotypes were more frequent in the fast progressing group B. Our results are in concordance with the study of Song et al., in which the CC genotype was linked to an unfavorable outcome in Japanese patients with IgAN.Citation12 We could reproduce the same association also in a cohort of patients with a different glomerulonephritis form (focal segmental glomerulosclerosis) [Bantis et al., abstract Su315 in the world congress of nephrology 2009 (www.wcn2009.org)].
The influence of aldosterone synthase gene C-344T polymorphism on the clinical course of glomerular diseases could be well understood by an upregulated aldosterone on tissue or plasma level. Aldosterone induces inflammation, fibrosis, mesangial cell proliferation, and podocyte injury in the kidney independent of its volume homeostasis and hemodynamic actions.Citation3 Aldosterone stimulates the phosphorylation and activation of epithelial growth factor receptor, which leads to activation of the MAPK (mitogen-activated protein kinases) pathways and proliferation of the mesangial cells.Citation13 The profibrotic action of aldosterone in the kidney is mainly mediated by transforming growth factor-β (TGF-β), whose expression is induced by aldosterone in cultured mesangial cells.Citation14 Furthermore, aldosterone increases the expression of intercellular adhesion molecule-1,Citation15 interleukin-6, and MCP-1 (monocyte chemoatrtactant protein-1),Citation16 which contribute to the progression of mesangial fibrosis and inflammation. Aldosterone acts directly also on podocytes leading to severe lesions in their cell architecture, denudation of the glomerular basement membrane, and subsequent development of hyalinosis, thrombosis, and synechia.Citation17 In anti-Thy-1 mesangioproliferative glomerulonephritis in particular, administration of aldosterone antagonists decreased mesangial cell proliferation, TGF-β levels, artery, glomerular, tubulointerstitial injury scores, and proteinuria.Citation18,Citation19 In humans, randomized studies have confirmed the beneficial effect of aldosterone antagonism on proteinuria reduction and attenuation of progression.Citation20,Citation21
In conclusion, this study suggests that the aldosterone synthase (CYP11B2) gene C-344T polymorphism is a risk factor for accelerated progression in Caucasian patients with IgAN. Even though our results are supported by similar findings in Japanese patients, larger prospective multicenter studies with standardized antihypertensive protocols are required to validate them.
ACKNOWLEDGMENT
Dr. Bantis was a recipient of a research scholarship of the German Academic Exchange Service (DAAD, A/99/13154, A/05/25663).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Praga M, Gutierrez E, Gonzalez E, Morales E, Hernandez E. Treatment of IgA nephropathy with ACE inhibitors: A randomized and controlled trial. J Am Soc Nephrol. 2003;14(6):1578–1583.
- Bantis C, Ivens K, Kreusser W, Koch M, Klein-Vehne N, Grabensee B, Influence of genetic polymorphisms of the renin-angiotensin system on IgA nephropathy. Am J Nephrol. 2004;24(2):258–267.
- Briet M, Schiffrin EL. Aldosterone: Effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6(5):261–273.
- Xue C, Siragy HM. Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension. 2005;46(3):584–590.
- Brand E, Chatelain N, Mulatero P, Fery I, Curnow K,, Jeunemaitre X, Structural analysis and evaluation of the aldosterone synthase gene in hypertension. Hypertension. 1998; 32(2):198–204.
- White PC, Slutsker L. Haplotype analysis of CYP11B2. Endocr Res. 1995;21(1–2):437–442.
- Pojoga L, Gautier S, Blanc H, Guyene TT, Poirier O, Cambien F, Genetic determination of plasma aldosterone levels in essential hypertension. Am J Hypertens. 1998; 11(7):856–860.
- Schunkert H, Hengstenberg C, Holmer SR, Broeckel U, Luchner A, Muscholl MW, Lack of association between a polymorphism of the aldosterone synthase gene and left ventricular structure. Circulation. 1999;99(17):2255–2260.
- Paillard F, Chansel D, Brand E, Benetos A, Thomas F, Czekalski S, Genotype-phenotype relationships for the renin-angiotensin-aldosterone system in a normal population. Hypertension. 1999;34(3):423–429.
- Hautanena A, Lankinen L, Kupari M, Janne OA, Adlercreutz H, Nikkila H, Associations between aldosterone synthase gene polymorphism and the adrenocortical function in males. J Intern Med. 1998;244(1):11–18.
- Tamaki S, Iwai N, Tsujita Y, Kinoshita M. Genetic polymorphism of CYP11B2 gene and hypertension in Japanese. Hypertension. 1999;33(1 Pt 2): 266–270.
- Song J, Narita I, Goto S, Saito N, Omori K, Sato F, Gender specific association of aldosterone synthase gene polymorphism with renal survival in patients with IgA nephropathy. J Med Genet. 2003;40(5):372–376.
- Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009;296(6):F1323–F1333.
- Lai L, Chen J, Hao CM, Lin S, Gu Y. Aldosterone promotes fibronectin production through a Smad2-dependent TGF-beta1 pathway in mesangial cells. Biochem Biophys Res Commun. 2006;348(1):70–75.
- Terada Y, Kuwana H, Kobayashi T, Okado T, Suzuki N, Yoshimoto T, Aldosterone-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. J Am Soc Nephrol. 2008;19(2):298–309.
- Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG, Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63(5): 1791–1800.
- Kretzler M, Koeppen-Hagemann I, Kriz W. Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Arch. 1994;425(2):181–193.
- Gullulu M, Akdag I, Kahvecioglu S, Filiz G, Savci V. Aldosterone blockage in proliferative glomerulonephritis prevents not only fibrosis, but proliferation as well. Ren Fail. 2006;28(6):509–514.
- Asai M, Monkawa T, Marumo T, Fukuda S, Tsuji M, Yoshino J, Spironolactone in combination with cilazapril ameliorates proteinuria and renal interstitial fibrosis in rats with anti-Thy-1 irreversible nephritis. Hypertens Res. 2004;27(12):971–978.
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006; 70(12):2116–2123.
- Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1(5):940–951.