Abstract
We examined the associations of circulating levels of pentraxin 3 (PTX3), monocyte chemoattractant protein-1 (MCP-1), and some other inflammatory mediators with cardiorenal syndrome. In advanced chronic kidney disease (CKD) patients (estimated glomerular filtration rate <30 mL/min/1.73 m2), the values of area under the curve of PTX3, tumor necrosis factor α, and high-sensitivity C-reactive protein for the detection of the association of cardiovascular disease (CVD) were 0.664, 0.507, and 0.318, respectively. In contrast, serum levels of MCP-1 were significantly higher in CKD patients than in control subjects independently of association with CVD.
Abstract
Both pentraxin 3 (PTX3) and monocyte chemoattractant protein-1 (MCP-1) are mediators of inflammation. They also appear to play critical roles in vascular endothelial dysfunction but their associations with cardiorenal syndrome remain largely unknown. The objective of this study was to examine their associations with cardiorenal syndrome. Circulating levels of PTX3, MCP-1, and some other biomarkers were evaluated in 134 patients with chronic kidney disease (CKD) and/or cardiovascular disease (CVD) and 55 age- and gender-matched subjects without CKD or CVD. Levels of PTX3, high-sensitivity C-reactive protein (hsCRP), and tumor necrosis factor α (TNFα) were significantly higher in CKD patients with CVD than in those without CVD. In advanced CKD patients (estimated glomerular filtration rate <30 mL/min/1.73 m2), the values of area under the curve of PTX3, TNFα, and hsCRP for the detection of the association of CVD were 0.664, 0.507, and 0.318, respectively. In contrast, serum levels of MCP-1 were significantly higher in CKD patients than in control subjects independently of association with CVD. PTX3, hsCRP, and TNFα, but not MCP-1 could predict the presence of CVD as a complication associated with CKD. Additionally, PTX3 might be a more sensitive marker for the association of CVD than hsCRP and TNFα in patients with advanced CKD.
INTRODUCTION
Cardiovascular morbidity and mortality is greatly increased in patients with chronic kidney disease (CKD). Besides traditional risk factors, including hypertension, hyperlipidemia, and diabetes mellitus, chronic inflammation observed in CKD patients is considered to be one of the important nontraditional risk factors.Citation1,Citation2 Inflammatory biomarkers such as high-sensitivity C-reactive protein (hsCRP), interleukin-6, tumor necrosis factor α (TNFα), serum amyloid P‐component (SAP), and fibrinogen have been well studied; especially, there are several associations with levels of hsCRP and cardiovascular disease (CVD) outcomes in patients with CKD.Citation2,Citation3
Although CRP and SAP are the prototypes of the short pentraxin family and mainly produced in the liver, pentraxin 3 (PTX3) is the prototype of the long pentraxin family and produced in peripheral tissues (e.g., endothelial cells, macrophages, T cells, smooth muscle cells) and is modulated partly by inflammatory mediators such as TNFα.Citation4–7 There are several evidences showing the link between PTX3 levels and ischemic heart disease. PTX3 levels increase rapidly in acute myocardial infarction and exhibit high levels in patients with unstable angina and those undergoing stenting.Citation8–10 In addition, CKD patients with or without hemodialysis treatment have higher plasma levels of PTX3 than those without CKD.Citation11,Citation12 There have been several studies reporting PTX3 levels with regard to cardiorenal syndrome. Plasma levels of PTX3 in patients on hemodialysis therapy are higher with coronary artery disease and peripheral artery disease than without these diseases.Citation12 Moreover, CKD patients with CVD who are not on hemodialysis treatment have higher concentrations of plasma PTX3 levels than their counterparts, and they have higher all-cause and cardiovascular mortality.Citation12,Citation13 Monocyte chemoattractant protein-1 (MCP-1) is one of the important chemotactic factors for monocyte and is released under inflammatory conditions. MCP-1 is regulated by some stimuli including TNFαCitation14–16 and is expressed by a variety of inflammatory activated cells such as endothelial cells, smooth muscle cells, and monocytes/macrophages and develops vascular atherosclerotic lesions.Citation17 An increase in plasma levels of MCP-1 in patients with unstable anginaCitation18 and an independent association between plasma levels of MCP-1 and clinical outcomes in patients with acute coronary syndromeCitation19 have been reported. On the other hand, circulating levels of MCP-1 are elevated in CKD patients including hemodialysis patients.Citation20,Citation21 However, unlike PTX3, the association between circulating levels of MCP-1 and cardiorenal syndrome is not well investigated. Both PTX3 and MCP-1 are associated with inflammation and atherosclerosis and their levels increase in both CKD and CVD patients. Thus, these two biomarkers are expected to have predictive value of cardiorenal syndrome, but the association and the role of them in CKD and/or CVD patients have not been well studied. The aim of this study was to investigate the circulating levels of PTX3 and MCP-1 and evaluate which of them could predict the presence of CVD in CKD patients.
MATERIALS AND METHODS
This study was performed at University of Miyazaki Hospital and Miyazaki Konan Hospital. Recruitment of the patients with CKD or CVD occurred from April 2008 through March 2009, who had admitted in the First Department of Internal Medicine, University of Miyazaki Hospital. A total of 134 patients (mean age 61.9 ± 14.8 years, 95 males) with CKD and/or CVD were enrolled. The exclusion criteria were clinical signs of acute inflammatory or infectious disease, malignancy, and patients on dialysis therapy at the time of evaluation. Blood samples of the patients were taken 7.9 ± 4.6 days after their admission.
These participants were classified as suffering from CKD if the estimated glomerular filtration rate (eGFR) was less than 60 mL/min/1.73 m2 (N = 86) or if they have proteinuria without decrease of eGFR (N = 21). Primary kidney disease of CKD patients were chronic glomerulonephritis in 31 patients (29.0%), diabetic nephropathy in 9 patients (8.4%), nephrosclerosis in 36 patients (33.6%), and others including secondary renal insufficiency in 15 patients (14.0%) and unknown in 16 patients (15.0%).
A total of 76 patients had CVD at the time of evaluation. Presence of CVD was defined as the following: ischemic heart disease diagnosed in cases where there were coronary artery stenoses by coronary angiography (N = 47, 61.8%), congestive heart failure or systolic dysfunction with ejection fraction below 50% (N = 11, 14.5%), valvular disease that needs surgical treatment (N = 11, 14.5%), history of cardiac arrest or shock (N = 5, 6.6%), aortic aneurysm or dissection (N = 3, 4.0%), stroke (N = 4, 5.3%), peripheral artery disease (N = 5, 6.6%), and a composite of these. The control subjects were matched to the patients with respect to age and gender (N = 55, age 58.6 ± 4.4 years, 44 males) to be used for comparative analyses of biochemical parameters.
All the participants were divided into four groups as follows: CKD(−)/CVD(−) as Group C (control group, N = 55), CKD(−)/CVD(+) as Group V (CVD group, N = 27), CKD(+)/CVD(−) as Group K (CKD group, N = 58), and CKD(+)/CVD(+) as Group KV (CKD group associated with CVD, N = 49).
Blood samples were immediately separated to plasma and serum within 30 min and samples were kept frozen at −80°C until assay. Plasma concentration of PTX3 was measured by enzyme-linked immunosorbent assay (ELISA) method (Perseus Proteomics, Inc., Tokyo, Japan). Serum concentrations of TNFα and MCP-1 were also determined by ELISA method (R&D Systems, Inc., Minneapolis, MN, USA). Plasma hsCRP levels were measured using high-sensitivity assays (Denka Seiken, Tokyo, Japan). eGFR was calculated according to the Japanese Society of Nephrology formula in 2008.Citation22 The ethical committees of University of Miyazaki Hospital and Miyazaki Konan Hospital approved this study and informed consent was obtained from all patients and controls.
Statistical Analysis
Data are expressed as mean ± SD. p < 0.05 was considered to be statistically significant. Comparison between two groups was assessed for continuous variables with the Student's unpaired t-test, Mann–Whitney test, or chi-square test, as appropriate. Differences among three or four groups were analyzed by analysis of variance (ANOVA) using one-way ANOVA, followed by Tukey honestly significant difference (HSD) test as a post hoc test if ANOVA was significant. Pearson product moment correlation was used to determine correlations of eGFR with other variables. For evaluation of the sensitivity and specificity of plasma levels of PTX3, TNFα, and hsCRP as predictor of the presence of CVD in CKD patients, a receiver operator characteristics (ROC) analysis was performed. Multivariate analysis was carried out on age, gender, and variables that were significantly different between CKD patients with and without CVD. Nonnormally distributed variables were log transformed before entering analysis. The statistical analysis was performed using JMP statistical software (version 8.0; SAS Institute, Japan).
RESULTS
Baseline characteristics of control subjects and patients with CKD and/or CVD are shown in . Plasma levels of PTX3 and serum levels of TNFα, hsCRP, and MCP-1 were significantly higher in patients with CKD and/or CVD compared with control subjects. Overall, in univariate analysis, TNFα (r = −0.49, p < 0.0001), PTX3 (r = −0.26, p < 0.001), hsCRP (r = −0.24, p < 0.001), and MCP-1 (r = −0.38, p < 0.0001) were significantly and negatively correlated with eGFR. Baseline characteristics of the four groups such as Groups C, V, K, and KV were shown in . As for traditional risk factors of CVD, the percentage of hypertension was significantly higher in the CVD group than in the control group. In the patients with CKD, age, the percentage of diabetes mellitus, and hypertension were significantly higher in patients with CVD than in those without CVD. The value of eGFR was significantly lower in CKD patients with CVD than in those without CVD. Contrary to our expectation, total cholesterol was lower in the subjects with CVD independently of CKD compared with the controls.
Table 1. Baseline characteristics of subjects without CKD or CVD and with CKD and/or CVD
Table 2. Baseline characteristics of four groups
Meanwhile, as for biomarkers, crude data of PTX3, TNFα, MCP-1, and hsCRP are shown in . Levels of TNFα and PTX3 were significantly higher in the CVD group and the CKD group compared with the control group. They were also higher in the CKD group with CVD than in those without CVD. Levels of hsCRP were not different between the CVD group and the control group, but were significantly higher in the CKD group with CVD than in those without CVD. In contrast, serum levels of MCP-1 were significantly higher in the CKD group than in the control group independently of association with CVD.
Table 3. Comparison of biomarker levels in the investigated participants
Moreover, considering the degree of eGFR in CKD patients with CVD, levels of TNFα and PTX3 were significantly higher in patients with eGFR < 30 mL/min/1.73 m2 than in those with eGFR ≥ 30mL/min/1.73 m2 (5.06 ± 2.85 vs. 3.40 ± 3.57 pg/mL, p < 0.01; 5.70 ± 3.36 vs. 3.80 ± 1.91 ng/mL, p < 0.05, respectively) but not hsCRP nor MCP-1 (). The ROC curve analysis showed that the area under the curve (AUC) for levels of PTX3 in advanced CKD patients (eGFR < 30 mL/min/1.73 m2) with CVD was 0.664. The plasma PTX3 cutoff value was 4.05 ng/mL, and its sensitivity and specificity were 71.4% and 41.4%, respectively (A). On the contrary, AUC for levels of TNFα and hsCRP in advanced CKD patients with CVD were 0.507 and 0.318, respectively (A and C). In the multivariate analysis, older age (odds ratio 1.24), greater body mass index (odds ratio 1.62), and greater levels of PTX3 (odds ratio 1.56) were independently associated with the presence of CVD in advanced CKD patients ().
Figure 1. Circulating levels of TNFα, PTX3, hsCRP, and MCP-1 in CKD patients with CVD with their eGFR ≥ 30 mL/min/1.73 m2 (N = 35) and <30 mL/min/1.73 m2 (N = 14).
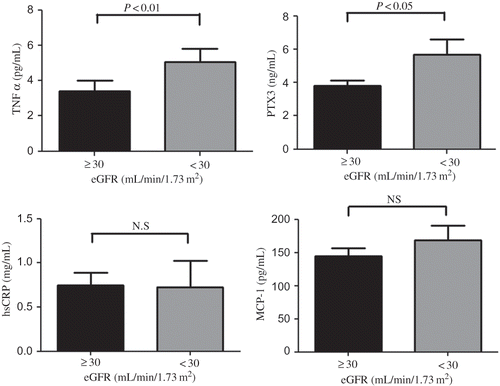
Figure 2. Receiver characteristic operating curves for differentiating the presence of CVD in advanced CKD patients (eGFR < 30 mL/min/1.73 m2) on the basis of the circulating levels of (A) PTX3, (B) TNFα, and (C) hsCRP (area under the curve = 0.664, 0.507, and 0.318, respectively).
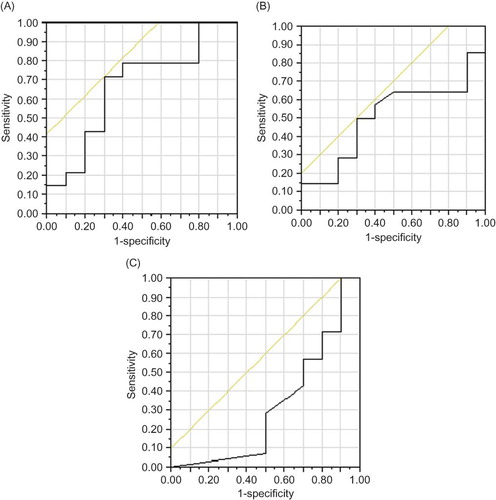
Table 4. Factors independently associated with the presence of CVD in advanced CKD patients (eGFR < 30 mL/min/1.73 m2)
DISCUSSION
In agreement with previous studies, PTX3 and MCP-1 levels were negatively correlated with eGFR.Citation13,Citation20 One of the main reasons of this result is probably due to the higher concentration of them in advanced CKD patients. These negative correlations could be explained by inadequate clearance of large molecular weight of them and enhanced synthesis and release upon stimulation in peripheral tissues including renal epithelial cells and mesangial cells.Citation13,Citation20,Citation23,Citation24 There might be another mechanism that explains our results in the inflammatory point of view.
This study shows that levels of PTX3 but not MCP-1 increase in CKD patients with CVD. Previous studies reported that plasma PTX3 levels were significantly higher in CKD patients with CVD than in those without CVD.Citation13 In addition, this study showed that levels of PTX3 were significantly higher in CKD patients with eGFR < 30 mL/min/1.73 m2, which corresponds to CKD stages 4 and 5 according to KDOQI Clinical Practice Guidelines for Chronic Kidney Disease than those with eGFR ≥ 30mL/min/1.73 m2. It is plausible that similar result was seen in levels of TNFα, as TNFα has been proved to be proinflammatory mediator that upregulates the expression of PTX3. As shown in ROC, in CKD patients with eGFR < 30 mL/min/1.73 m2, circulating levels of PTX3 might play a more important role in detecting the presence of CVD than levels of hsCRP, which have been reported to be an independent predictor of cardiorenal syndrome.Citation3 PTX3 is the prototype of the long pentraxin family produced in a variety of tissues and cells at sites of inflammation.Citation4,Citation25,Citation26 Inflammatory response of PTX3 is closely linked to vascular endothelial dysfunction. Recent studies reported that PTX3 is strongly expressed in atherosclerotic lesions, primarily in monocytes, macrophages, endothelial cells, and smooth muscle cells.Citation27,Citation28 Moreover, several studies demonstrated that PTX3 is induced in vascular smooth muscle cells by atherogenic-modified low-density lipoprotein and is present in atherosclerotic lesions.Citation27,Citation29 PTX3 inhibits FGF2-dependent activation of smooth muscle cells.Citation30 Therefore, it could be said that PTX3 plays a role in the defense mechanism of CVD. On the other hand, PTX3 induced in endothelial cells might promote thrombogenesis and vascular ischemia.Citation31 In advanced CKD patients, higher concentration of plasma PTX3 might potentiate CVD to occur through these inflammatory responses mentioned above. Our study suggests that in advanced CKD patients, PTX3 might have more information and role in predicting CVD than hsCRP.
On the other hand, unlike PTX3 levels, there was no difference of MCP-1 levels between CKD patients with and without CVD. MCP-1 is expressed in endothelial cells, smooth muscle cells, and macrophages in atherosclerotic lesions and induces endothelial dysfunction, thrombosis, and angiogenesis and develops atherosclerosis and plaque destabilization.Citation17,Citation32 Elimination of the MCP-1 pathway decreases atheroma formation and inhibits the development of atherosclerosis in mice.Citation33 These findings support that MCP-1 might progress in a positive feedback loop to enhance atherosclerotic changes at the lesion of vascular inflammation.Citation32 MCP-1 acts as vascular inflammatory mediator by developing atherosclerosis. In addition, uremic toxicity induces the expression of cytokines and adhesion molecules including MCP-1.Citation20 Thus, increasing levels of MCP-1 in CKD patients with CVD could be induced by uremic milieu more than by vascular inflammation. In other words, cardiovascular inflammation has a less effect on serum MCP-1 concentration in CKD patients with CVD.
We report here for the first time the comparison of the levels of PTX3 and MCP-1 in the presence of CVD in CKD patients. Compared with hsCRP, which has been considered to be reliable inflammatory marker of CVD in CKD patients, the higher levels of PTX3 might indicate the comorbid condition of CVD in advanced CKD patients or in patients with CKD stages 4 and 5. On the other hand, the comorbid condition of CVD in CKD patients does not affect the levels of MCP-1.
ACKNOWLEDGMENTS
We express appreciation to Drs. Hiroko Fukae and Yurika Kawano and Mses. Kumiko Kawakita and Mina Sagara for their help in measuring circulating levels of mediators, and to Drs. Keiichi Fukudome and Osamu Ogawa for their collaborative assistance.
Declaration Of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sarnak MJ, Levey AS, Schoolwerth AC, Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169.
- Roberts MA, Hare DL, Ratnaike S, Ierino FL. Cardiovascular biomarkers in CKD: Pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis. 2006;48:341–360.
- Menon V, Greene T, Wang X, C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772.
- Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: From C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28:1–13.
- Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366.
- Introna M, Alles VV, Castellano M, Cloning of mouse PTX3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–1872.
- Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493.
- Peri G, Introna M, Corradi D, PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641.
- Inoue K, Sugiyama A, Reid PC, Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol. 2007;27:161–167.
- Kotooka N, Inoue T, Fujimatsu D, Pentraxin3 is a novel marker for stent-induced inflammation and neointimal thickening. Atherosclerosis. 2008;197:368–374.
- Suliman ME, Yilmaz MI, Carrero JJ, Novel links between the long pentraxin 3, endothelial dysfunction, and albuminuria in early and advanced chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:976–985.
- Boehme M, Kaehne F, Kuehne A, Pentraxin 3 is elevated in hemodialysis patients and is associated with cardiovascular disease. Nephrol Dial Transplant. 2007;22:2224–2229.
- Tong M, Carrero JJ, Qureshi AR, Plasma pentraxin 3 in patients with chronic kidney disease: Associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2007;2:889–897.
- Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990;11:97–101.
- Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990;136:1229–1233.
- Sica A, Wang JM, Colotta F, Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990;144:3034–3038.
- Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis. 1999;147:213–225.
- Hojo Y, Ikeda U, Takahashi M, Shimada K. Increased levels of monocyte-related cytokines in patients with unstable angina. Atherosclerosis. 2002;161:403–408.
- de Lemos JA, Morrow DA, Sabatine MS, Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695.
- Stinghen AE, Goncalves SM, Martines EG, Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin Pract. 2009;111:c117–c126.
- Papayianni A, Alexopoulos E, Giamalis P, Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in hemodialysis patients: Association with inflammation, dyslipidaemia, and vascular events. Nephrol Dial Transplant. 2002;17:435–441.
- Imai E, Horio M, Watanabe T, Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–630.
- Nauta AJ, de Haij S, Bottazzi B, Human renal epithelial cells produce the long pentraxin PTX3. Kidney Int. 2005;67:543–553.
- Bussolati B, Peri G, Salvidio G, Verzola D, Mantovani A, Camussi G. The long pentraxin PTX3 is synthesized in IgA glomerulonephritis and activates mesangial cells. J Immunol. 2003;170:1466–1472.
- Mantovani A, Garlanda C, Bottazzi B, The long pentraxin PTX3 in vascular pathology. Vascul Pharmacol. 2006;45:326–330.
- Bottazzi B, Garlanda C, Salvatori G, Jeannin P, Manfredi A, Mantovani A. Pentraxins as a key component of innate immunity. Curr Opin Immunol. 2006;18:10–15.
- Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–e14.
- Savchenko A, Imamura M, Ohashi R, Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol. 2008;215:48–55.
- Klouche M, Peri G, Knabbe C, Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis. 2004;175:221–228.
- Camozzi M, Zacchigna S, Rusnati M, Pentraxin 3 inhibits fibroblast growth factor 2-dependent activation of smooth muscle cells in vitro and neointima formation in vivo. Arterioscler Thromb Vasc Biol. 2005;25:1837–1842.
- Napoleone E, Di Santo A, Bastone A, Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: A novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol. 2002;22:782–787.
- Egashira K:. Molecular mechanisms mediating inflammation in vascular disease: Special reference to monocyte chemoattractant protein-1. Hypertension. 2003;41:834–841.
- Gu L, Okada Y, Clinton SK, Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281.