Abstract
Immune function disorders are common during acute renal failure (ARF), but the mechanisms are unknown. As the spleen is the largest organ of the immune system, we aimed to observe if there are morphological changes in the spleen in rabbits with ARF. In addition, we tried to explore its mechanism from the perspective of oxygen free radicals, nitric oxide (NO), myeloperoxidase (MPO), and membrane pump activities. ARF animal models were established by either hypodermic injection of 1.3 mL/kg bw 1% HgCl2 or intramuscular injection of 10 mL/kg bw 50% glycerin. Animals were divided into 12 h, 24 h, and 48 h treatment groups with six rabbits in each group. Compared with control animals, congestion was found in the spleen and splenic trabeculae were increased in the two ARF model groups at multiple time points. The malonaldehyde, NO, nitric oxide synthase, and MPO levels in the ARF models were increased compared with the control group at 24 h or 48 h, and the superoxide dismutase and adenosine triphosphatase activities were significantly lower than the levels in the control group at multiple time points. These indices of free radical damage were induced gradually with ARF development, and there were statistically significant differences at different time points. These data suggested that histological damage of spleen during ARF may lead to immune disorders, which might be related to free radical injury, NO excessive release, polymorphonuclear neutrophils (PMN) sequestration, and membrane pump dysfunction.
INTRODUCTION
The spleen is the largest immune organ of the human body and accounts for 25% of the total lymphoid tissues. It contains large amounts of lymphocytes and macrophages and constitutes the center of cellular immunity and humoral immunity. The immune functions in the body can be controlled by the spleen through a variety of mechanisms. Therefore, the regulation of the spleen functions is of significance for both cellular and humoral immunity.Citation1,Citation2 It has been reported that acute renal failure (ARF) can induce the disturbance of humoral immunity, which is characterized by excessive release and activation of immunoglobulin and complement.Citation3 The disorder of the internal environment resulting from ARF such as metabolic waste accumulation, water–electrolyte disturbance, metabolic acidosis, and so on may be one of major factors leading to immunity dysfunction. As a critical immunity organ, the role that the spleen plays in the process of immune dysfunction after ARF remains unclear. Thus, the aim of this study was to observe the morphological changes in the spleen in ARF rabbits and explore its mechanism in terms of free radical, nitric oxide (NO), myeloperoxidase (MPO), and membrane pump activities, and into the role of spleen on immune function disorders during ARF.
METHODS
Animals and Grouping
Adult healthy New Zealand rabbits weighing 2–3 kg (purchased from Hebei New Zhangjiakou Pharmaceutical Co., Ltd., Zhangjiakou, China) were used in this study after a minimum 5-day acclimation period. Animals were housed under barrier-sustained conditions with 12 h light/dark cycles and free access to standard laboratory food and water. All experiments were performed in accordance with the recommendations of the “Guide for the Care and Use of Laboratory Animals” and approved by the Medical School Animal Use and Care Committee of Hebei North University.
Rabbit ARF Models and Sample Preparation
Rabbits were randomly assigned to three groups: an untreated control group, a HgCl2-treated group, and a glycerin-treated group. ARF animal models were established, respectively, by hypodermic injection of 1% HgCl2 at 1.3 mL/kg bw in the HgCl2-treated group or by intramuscular injection of 50% glycerin at 10 mL/kg bw in the glycerin-treated group as described previously,Citation4–6 and all animals in each group were further divided into 12 h, 24 h, and 48 h subgroups with each consisting of six rabbits. Our previous work had documented that rabbits receiving the equivalent volume of physiological saline instead of HgCl2 or glycerin have no changes in their physiological indexes;Citation4–6Citation6 therefore, six healthy rabbits served as the control group.
All animals in the 12 h, 24 h, and 48 h subgroups were anesthetized with 1 g/kg urethane at 12 h, 24 h, and 48 h after administration of HgCl2 or glycerin, respectively, and samples of blood and tissue were harvested for serological and biochemical analysis. Blood samples were drawn from the carotid artery and centrifuged at 850 ×g at 4°C for 20 min, and serum samples were stored at −70°C until serological assays were performed. Part of the spleen tissue was harvested in a fixed position and fixed in neutral buffered formalin for morphological observation; another piece of spleen tissue was homogenized in 1:9 (w/v) physiological saline for 30 s and then centrifuged at 850 ×g at 0–4°C for 10 min. The supernatant fluid was frozen at −70°C for further biochemical assays.
Renal Function Assessment
Blood urea nitrogen (BUN) and creatinine (Cre) levels in serum, which were used to assess renal function, were determined using a specific analysis kit (Shanghai Fosun Long March Medical Science Co., Ltd., Shanghai, China) using an automatic biochemical analyzer (Aeroset, Chicago, USA).
Spleen Histological Evaluation
After the spleen samples were fixed in 10% buffered methanol and embedded in paraffin for routine histological examination, hematoxylin and eosin staining was performed for histological diagnosis, morphological alterations were observed by light microscopy (BH-2, Olympus, Tokyo, Japan), and pictures were taken using a digital camera (4500, Nikon, Tokyo, Japan) from 10 randomly chosen areas per sample. All morphological examinations were done by a forensic pathologist without prior knowledge of experimental conditions.
Oxygen Free Radical Detection
As a marker of oxygen free radical activity in spleen homogenate, we measured tissue levels of malonaldehyde (MDA) using modified thiobarbituric acid (TBA) assayCitation7,Citation8 according to the instructions provided by the manufacturer (Jiancheng Biotechnology, Nanjing, China). Homogenate (0.1 mL) was mixed with 0.1 mL dehydrated alcohol, 0.1 mL TBA, and 4.0 mL developer, maintained in a water bath at 95°C for 40 min, cooled in running water, and centrifuged at 1200 ×g for 10 min. The supernatant was obtained and the absorbance was measured at 532 nm and the amount of MDA calculated as nanomoles per milligram of protein.
The activity of superoxide dismutase (SOD) in spleen homogenate was measured using xanthine oxidaseCitation9 according to the instructions provided by kit manufacturer (Jiancheng Biotechnology). Homogenate (0.1 mL) was mixed with 1.4 mL xanthine oxidase and maintained in a water bath at 37°C for 40 min. Developer (2.0 mL) was added and absorbance measured at 550 nm following a 10 min incubation at room temperature. Results are expressed as units of SOD per milligram of protein.
The total protein content in the tissue was determined by Coomassie blue:Citation10 homogenate (1%, 0.05 mL) was mixed with 3.0 mL Coomassie blue CBBG250 (Tokyo, Japan) and left at room temperature for 10 min. Shade selection was performed at 595 nm.
Nitrate (NO2−/NO3−) and Nitric Oxide Synthase Assays
Given that NO is highly labile, NO2–/NO3– was used as a surrogate marker for NO content using a nitrate reductase methodCitation11 kit (Jiancheng Biotechnology) according to manufacturer's protocol. Homogenate (0.1 mL) was mixed with 0.7 mL nitrate reductase and maintained at 37°C for 40 min. The supernatant (0.5 mL) was obtained and developer (0.6 mL) was added. The absorbance was measured at 532 nm following a 10 min incubation. Results were shown as micromoles of NO per gram of protein.
Nitric oxide synthase (NOS) is one of major enzymes which induces the NO synthesis and release. Therefore, for observing the reason of NO content changes, the activity of NOS was measured using the chemical chromogenic methodCitation12 according to the manufacturer's directions of kits (Jiancheng Biotechnology). Homogenate (0.1 mL) was mixed with 0.2 mL substrate buffer solution (20 µL arginine solution, 20 µL nicotinamide adenine dinucleotide phosphate, and 0.8 µL 4-amino-5-methylamino-2′,7′-difluorofluorescein), 0.01 mL accelerant, and 0.1 mL developer, maintained in a water bath at 37°C for 15 min, and remixed with 0.1 mL clearing medium and 2.0 mL stop buffer. The absorbance was measured at 530 nm. Results were shown as units of NOS per gram of protein.
MPO Activities Assays
MPO activities in spleen homogenate were measured with the hydrogen peroxide methodCitation13 according to the instructions provided by the manufacturer of the MPO kit (Jiancheng Biotechnology). Homgenate (0.2 mL) was mixed with 0.2 mL hydrogen peroxide and 3.0 mL developer, maintained in a water bath at 37°C for 30 min, remixed with 0.05 mL hydrogen donor (AH2), and maintained in a water bath at 60°C for 10 min. The absorbance was measured at 460 nm. One unit of MPO activity represents the amount of enzyme that will reduce 1 µmol/min of hydrogen peroxide. Results were shown as units of MPO per gram of protein.
Membrane Pump Assays
Na+–K+–ATPase, Ca2+–ATPase, Mg2+–ATPase, and Ca2+–Mg2+–ATPase activities were measured with the fixing phosphorus methodCitation14 using a commercially available kit (Jiancheng Biotechnology). Spleen homogenate (0.2 mL) was mixed with 0.3 mL adenosine triphosphatase (ATPase) and centrifuged at 1200 ×g for 10 min. The supernatant (0.2 mL) was added to 2.0 mL fixing phosphorus buffer solution [1N sulfuric acid, 0.5% (w/v) ammonium molybdate, and 0.02% (w/v) SnCl2], maintained in a water bath at 37°C for 30 min, and cooled at room temperature. The absorbance was measured at 660 nm. The quantity of inorganic phosphorus was considered as the standard of ATPase activity. One unit of ATPase activity represents the amount of enzyme that will produce 1 µmol/h of organic phosphorus from the hydrolysis of ATP to ADP. Results were shown as units of ATPase per milligram of protein.
Statistical Analysis
All data are presented as mean ± SD. The statistical analysis was performed using SPSS software 16.0. One-way ANOVA analysis was used between groups and Student-Newman-Keuls (SNK) test was used within groups. p-Values < 0.05 were considered statistically significant.
RESULTS
Indicator of Renal Function
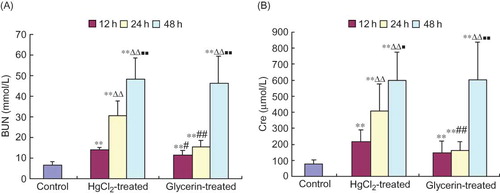
BUN and Cre levels in both HgCl2-treated and glycerin-treated animal groups tended to gradually enhance with time, with measurements at 12 h, 24 h, and 48 h being significantly higher than those in the control group (p < 0.01, ).
Figure 1. Changes of biochemical indexes of renal function in rabbits (mean ± SD, n = 6).
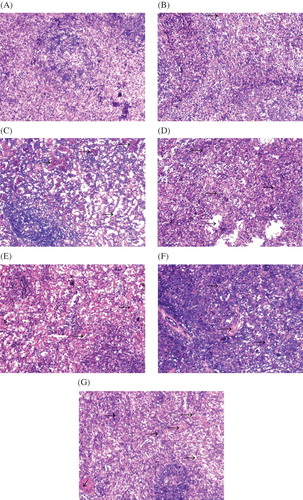
Histological Alterations in Spleen
The configuration and structure of spleen tissues was observed as normal in the control animals (). In the HgCl2-treated group, the architecture of the spleen tissues remains almost normal but occasionally is accompanied by slight congestion at 12 h time point (), by 24 h this congestion becomes rather serious (), and at the 48 h time point trabeculae lienis increases gradually and lenic sinus expands mildly (). In the glycerin-treated group, at the 12 h time point the congestion is present in the spleen tissues () and at the 24 h and 48 h time points the congestion and trabeculae lienis gradually increased; at the same time points, spleen sinus expansion appeared ( and G).
Figure 2. Change of spleen histomorphology in two rabbit models of ARF (hematoxylin and eosin staining, ×100). (A) Control group; (B) HgCl2-treated group at 12 h; (C) HgCl2-treated group at 24 h; (D) HgCl2-treated group at 48 h; (E) glycerin-treated group at 12 h; (F) glycerin-treated group at 24 h; (G) glycerin-treated group at 48 h. Arrows show congestion in C, E, and F and trabeculae lienis in D and G.
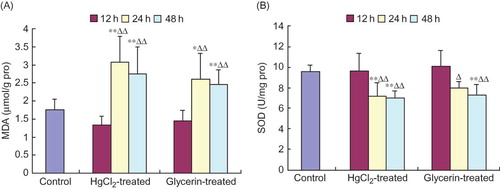
Free Radical Index Alterations in Spleen Homogenate
At 24 h and 48 h after animals were subjected to HgCl2 or glycerin extrusion, the contents of MDA in spleen homogenates increased significantly compared with control animals and, moreover, were elevated obviously when compared with those of at the 12 h time point within a same group (p < 0.01, p < 0.05, respectively, ). At 24 h and 48 h in the HgCl2-treated group and at 48 h in the glycerin-treated group, the activities of SOD in spleen homogenates were significantly lower than those of the control group. At 24 h and 48 h in the two treated groups, the activities of SOD declined markedly compared with those of the 12 h time point within the same group (p < 0.01, p < 0.05, ). No significant differences were found between the two treated groups at 12 h and the control group (p > 0.05).
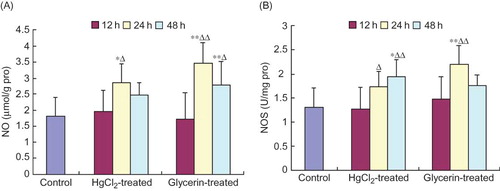
NO and NOS Alterations in Spleen Homogenate
The levels of NO at 24 h in the HgCl2-treated group and at 24 h and 48 h in the glycerin-treated group and the activities of NOS at 48 h in the HgCl2-treated group and at 24 h in the glycerin-treated group increased significantly when compared with those of the control group. Moreover, the levels of NO at 24 h in the HgCl2-treated group and at 24 h and 48 h in the glycerin-treated group and the activities of NOS at 48 h in the HgCl2-treated group and at 24 h in the glycerin-treated group were dramatically higher than those at 12 h time point within a same group (p < 0.01, p < 0.05, ).
MPO Activity Alterations in Spleen Homogenate
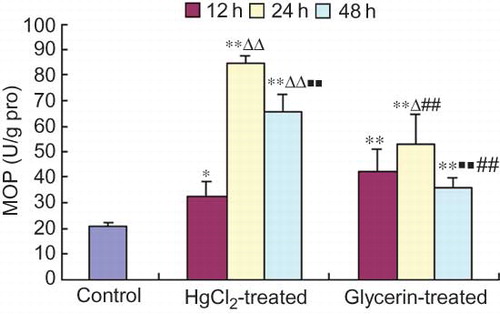
The activity of MPO in spleen homogenate was significantly higher than that of the control group at each time point sampled of the HgCl2- and the glycerin-treated animals. MPO activity at 24 h was higher than that of at 12 h within both the treated groups. In the HgCl2-treated group, the MPO activity reached the highest level at 24 h and then declined gradually at 48 h. In the glycerin-treated group, the MPO activity at 48 h was lower than that at 24 h within the group. Besides, the MPO activity at 24 h and 48 h in the glycerin-treated group was significantly lower than that at the corresponding time points in the HgCl2-treated group ().
Figure 5. Changes of MPO activity in spleen homogenate of rabbit models of ARF (mean ± SD, n = 6).
ATPase Activity Alterations in Spleen Homogenate
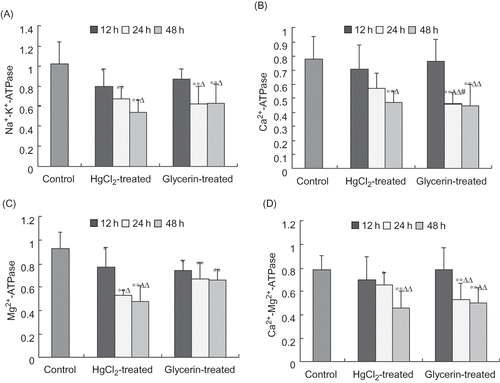
The activity of Na+–K+–ATPase in spleen homogenate declined significantly at each time point of the HgCl2-treated group and at 24 h and 48 h in the glycerin-treated group compared with the control group; moreover, the activity was lower at 48 h in the HgCl2-treated group and at 24 h and 48 h in the glycerin-treated group than that of at 12 h within a same group significantly. The activity of Ca2+–ATPase of spleen at 48 h in the HgCl2-treated group and at 24 h and 48 h in the glycerin-treated group declined significantly compared with that of the control group and at 12 h within the group. The activity of Mg2+–ATPase of spleen tissues at each time point of the HgCl2- and the glycerin-treated group was decreased significantly compared with that of the control group. The activity of Ca2+–Mg2+–ATPase has similar tendency to Mg2+–ATPase, Ca2+–ATPase, and Na+–K+–ATPase during the development of ARF (see ).
Figure 6. ATPase activities were significantly suppressed in the spleen homogenate of rabbit models of ARF (µmolPi/mg.pro/h, mean ± SD, n = 6).
DISCUSSION
In this study, we found that morphological lesions of the spleen had appeared in two ARF animal models, manifesting with congestion or increase of trabeculae lienis at different time points. The formation of congestion may be involved in the hyperviscosity of plasmaCitation15 and waste product retention, which may lead to the pathogenesis of microcirculatory disturbances and morphological changes in spleen tissues. During the development of ARF, trabeculae lienis was increased gradually, suggesting the presence of hyperplasia in the spleen. The cause of the hyperplasia of the fibrous tissue may be either the poison accumulation or the hemorheological abnormalities. Immune dysfunction has been proposed as one of major mechanisms to the development of systemic infections and even multiple organ dysfunction syndrome due to ARF. This study was focused on spleen, a pivotal immune organ, in the process of the immune disturbance after ARF.
The results of this study indicated that in the progression of ARF caused by mercury poisoning or glycerin extrusion, the MPO activities in spleen were both significantly higher than those in the control group at multiple time points. MPO activities had a slight decline at 48 h; however, the statistical difference still remained when compared with the control group. MPO is an enzyme that is found predominantly in the azurophilic granules of PMN. Tissue MPO activity correlates significantly with the number of PMN in insulted tissuesCitation16 and is frequently utilized to estimate tissue PMN. The results suggest that during the process of ARF, PMN in spleen tissue increased robustly, which may be involved in the hemorheological disturbancesCitation15 or the overexpression of intercellular adhesion molecules.Citation17 The PMN sequestrated in spleen tissues can release proinflammatory mediators and therefore arouse inflammatory cascade effects. These events will exacerbate the excessive production of free radical species. The increases of radical production are responsible for the injury of cell membrane in spleen tissues, which can trigger systemic inflammatory responses. The amplification of inflammatory responses can accentuate further injury caused by free radical activity and thus sets up a vicious circle of tissue damage. The present studies show that in the early process of ARF development (at 12 h after administration), no significant differences were observed in MDA content and SOD activities, which are considered as a reliable markers of oxygen free radical injury, as well as the nitrite content and NOS activities. With the development of ARF, differences gradually emerged in these indicators of spleen tissues, manifesting the increase of MDA and nitrite contents and NOS activities associated with the decline of SOD activities. Our study showed that the PMN sequestration induced by hemorheological abnormalities after ARF combined with the release of NO and MDA results in the injury of spleen tissue and the subjected tissues can further aggravate the production of oxygen free radicals and NO, which exacerbates the lipid peroxidation and inflammatory response at involved tissues on the one hand. On the other hand, the disturbance of internal environment and metabolic waste accumulation can also exacerbate spleen injury after ARF and increase the content of free radicals and NO. Simultaneously, immune dysfunction due to spleen injury after ARF can worsen the damage caused by systemic inflammatory response and lead to a predisposition toward certain infections. These results suggest that the PMN infiltration and excessive release of free radicals are involved in the mechanism of spleen injury on various flanks during the process of ARF.
This study showed that the activities of Na+–K+–ATPase, Ca2+–ATPase, Mg2+–ATPase, and Ca2+–Mg2+–ATPase in spleen tissues from ARF caused by two different factors decreased significantly when compared with those from the control group at multiple time points. ATPase is a membrane-bound protein found in all cells and its function is to maintain a chemical and electrical gradient across the cell membrane.Citation18,Citation19 The energy maintaining this electrochemical gradient comes from the hydrolysis of ATP to ADP. It is the energy stored in this gradient that drives other substances' membrane transport, cotransport, and exchange systems; allows the movement of cations, anions, amino acids, and glucose across the cell membrane; and maintains vital cellular functions including membrane electrical potential, cell volume, and intracellular Na+, K+, and Ca2+ concentration. The degree of cellular injury is closely related to the activity of ATPase.Citation18,Citation19 Any substance or clinical condition that alters the normal ATPase activity of cytomembrane will suppress the transportation of ions between inside and outside of these cells,Citation20 change the electrochemical gradient, and will lead to a wide array of cellular dysfunction that will ultimately aggravate the dysfunction and morphologic damage of organs. The present data also imply that the suppression of membrane pump activity is a pathogenesis of spleen injury in the process of ARF development. Besides the direct injury of poisonous substances, the severe derangement of internal environment such as water–electrolyte disturbance, metabolic wastes accumulation, and energy metabolic dysfunction may be responsible for this pathogenesis.
It is worth noting that this study was carried out using two different animal models of ARF, and the results were similar for the two models. Mercury poisoning is a representative model of ARF due to acute tubular necrosis caused by the action of heavy metals, organic solvents, and other toxins. After crush syndrome, ARF is induced by myoglobin blocking and hypoxic ischemic injury of renal tubule as well as the renal toxicity of myoglobin. From the perspective of etiology, mercury is usually one of the exogenous toxins, and myoglobins after crush syndrome caused by glycerin are one of endogenous injury. So, mercury is one of the cytoplasmic toxins and the injury mechanisms of renal tubule are different from that of myoglobin to some extent.Citation21 The results of this study indicate that spleen injury was present in both ARF induced by exogenous mercury and endogenous myoglobin, which suggests that the spleen injury is induced neither by HgCl2 nor glycerin purely. These results imply that the internal environment disorders following ARF may be one of the main reasons of spleen injury, which may be the common image of immunity dysfunction in the pathogenesis of ARF. Moreover, the indices of MPO, free radical, NO, and ATPase of spleen in ARF models induced by two different factors showed a similar tendency, which also suggested that the spleen injury is a general character in the pathogenesis of ARF.
Declaration of interest
: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
This study was supported by a grant from a key scientific and technological project of Hebei Province (06276102D-17), the Scientific Research Program of Education Department in Hebei Province (2006306); the Scientific Research Program of Zhangjiakou City (061159). No benefits in any form have been received or will be received from a commercial association related directly or indirectly to the subject of this article.
REFERENCES
- Maurya R, Kumar R, Prajapati VK, Human visceral leishmaniasis is not associated with expansion or accumulation of Foxp3+ CD4 cells in blood or spleen. Parasite Immunol. 2010;32(7):479–483.
- Semaeva E, Tenstad O, Skavland J, Access to the spleen microenvironment through lymph shows local cytokine production, increased cell flux, and altered signaling of immune cells during lipopolysaccharide-induced acute inflammation. J Immunol. 2010;184(8):4547–4556.
- Zhao YQ, Hou YL, Zhao ZG, Effects of the change of humoral immunity function in rabbits with acute renal failure. J Hebei North Univ. 2004;21(6):1–3.
- Zhao ZG, Niu CY, Hou YL, Changes in myocardium enzyme in rabbits with acute renal failure. Chin Crit Care Med. 2004;16(2):109–110.
- Zhao ZG, Niu CY, Hou YL, Effects of free oxygen radical and nitric oxide in multiple organ dysfunction syndrome induced by acute renal failure. Chin Crit Care Med. 2004;16(12):756–759.
- Zhao ZG, Niu CY, Zhang YP, Pancreatic injury in rabbit with acute renal failure. Ren Fail. 2009;31(10):977–981.
- Rochette L, Tatou E, Vergely C, Regional heterogeneity of decreased myocardial norepinephrine and increased lipid peroxidation levels in patients with end-stage failing heart secondary to dilated or ischemic cardiomyopathy. J Heart Lung Transplant. 2008;27(7):767–774.
- Zhao ZG, Niu CY, Shang AM, Mesenteric lymph reperfusion may exacerbate brain injury in a rat model of superior mesenteric artery occlusion shock. Neural Regen Res. 2010;5(9):683–689.
- Morild E, Olmheim JE. Pressure variation of enzymatic reaction rates: IV. Xanthine oxidase and superoxide dismutase. Physiol Chem Phys. 1981;13(6):483–491.
- Bautista J, Corpas R, Ramos R, Brain mitochondrial complex I inactivation by oxidative modification. Biochem Biophys Res Commun. 2000;275(3):890–894.
- Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141(7):2407–2412.
- Squadrito F, Altavilla D, Squadrito G, Recombinant human erythropoietin inhibits iNOS activity and reverts vascular dysfunction in splanchnic artery occlusion shock. Br J Pharmacol. 1999;127(2):482–488.
- Anderson BO, Brown JM, Shanley PF, Marginating neutrophils are reversibly adherent to normal lung endothelium. Surgery. 1991;109(1):51–61.
- Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na, K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation. 2000;102(24):3009–3014.
- Zhao ZG, Niu CY, Hou YL, Changes of coagulation function and hemorheological indexes of acute renal failure in rabbits. Chin J Microcirc. 2003;13(1):25–26.
- Niu CY, Zhao ZG, Ye YL, Mesenteric lymph duct ligation against renal injury in rats after hemorrhagic shock. Ren Fail. 2010;32(5):584–591.
- Dehne MG, Sablotzki A, Mühling J, Evaluation of sE-Selectin and sICAM-1 as parameters for renal function. Ren Fail. 2008;30(7):675–684.
- Murphy E, Eisner DA. Regulation of intracellular and mitochondrial sodium in health and disease. Circ Res. 2009;104(3):292–303.
- Billimoria FR, Dave BN, Katyare SS. Neonatal hypothyroidism alters the kinetic properties of Na+, K+-ATPase in synaptic plasma membranes from rat brain. Brain Res Bull. 2006;70(1):55–61.
- Garcés EO, Victorino JA, Veronese FV. Anticoagulation in continuous renal replacement therapies (CRRT). Rev Assoc Med Bras. 2007;53(5):451–455.
- Zhao ZG, Niu CY, Zhang YP, The mechanism of myocardium and pancreas injury in rabbits with acute renal failure might be related to myeloperoxidase and membrane pump activities. Ren Fail. 2010;32(10):1216–1222.