Abstract
Background: This study was designed to use carnitine for preventing deposition of end products of lipid peroxidation in rat models in the prevention of ischemia–reperfusion (IR) damage frequently seen following operations of infrarenal abdominal aorta (AA). Methods: Forty male rats of Sprague-Dawley type were evenly (n = 8) randomized to five groups: sham laparotomy (SHAM), carnitine control (CC), aortic IR (AIR), AIR + low-dose carnitine (AIR+LDC), and AIR + high-dose carnitine (AIR+HDC). Results: Compared to other groups, serum creatinine levels of AIR group were significantly higher. Also tissue malondialdehyde (MDA) levels of AIR group were significantly higher compared to SHAM, CC, and AIR+HDC groups. In histopathological examination, although tubular necrosis atrophy and tubular degeneration observed in AIR group showed regression with low-dose carnitine, tubular necrosis atrophy, tubular degeneration, glomerular damage, and vascular congestion thrombosis decreased with high-dose carnitine. Total score of histological damage was significantly higher in AIR, AIR+LDC, and AIR+HDC groups compared to SHAM and CC groups. Moreover, total score of histological damage was significantly lower in AIR+HDC group than AIR+LDC group. Conclusions: In this study, we showed carnitine can partially prevent renal damage in infrarenal AIR models of rats. This result may open new prospects to us in the prevention of renal IR damage during surgery of aorta.
INTRODUCTION
Ischemia–reperfusion (IR) damage seen during the surgery of abdominal aorta (AA) is a complex event and is observed not only in lower extremities but also in distant tissues and organs like lungs, kidneys, heart, and liver.Citation1,Citation2 Acute kidney injury (AKI) is one of the cardinal distant tissue damage observed in the surgery of AA and also called myonephrotic-metabolic syndrome.Citation3 Following elective surgery of AA, acute renal dysfunction is still frequently seen (15–22%) but AKI is relatively rare (1.8–4%).Citation4,Citation5 However, mortality rate is still higher with AKI (66%).Citation6 Generally, there is a common mechanism in organ damages observed following infrarenal aortic IR (AIR) and one of the underlying etiological factors is claimed to be ‘washout’ phenomenon. The role of many chemical mediators released into systemic circulation during reperfusion and possible microembolisms have been subject to some studies.Citation7–9 Infrarenal clamping of aorta has been shown to decrease renal blood flow especially in patients who postoperatively developed renal failure.Citation10–12 This clamping procedure can cause vasoconstriction in renal arteries by forming turbulent flow in aorta in level of renal arteries.Citation12 Cross-clamping, even infrarenal level, changes intrarenal blood flow patterns and less blood flows to cortical nephrons.Citation13 Also cross-clamping in any level of aorta causes a decrease in cardiac output. Mediators released because of systemic inflammatory response syndrome following IR deepen tissue damage in kidneys by causing vasoconstriction and redistribution of renal blood flow.Citation14 Although basic mechanism in infrarenal AIR damage is reperfusion, ischemia also contributes to this mechanism to some degree. Although many pharmacological and surgical methods have been developed to decrease this kind of damage, the problem could not be solved completely.
Carnitine is an antioxidant agent preventing deposition of end products of lipid peroxidation.Citation15,Citation16 Carnitine increases mitochondrial energy production which decreases in ischemic process by transfer of fatty acids and inducing oxidation of fatty acids in mitochondria. Consequently, more ATPs are produced and production of lactic acid, acidosis, and accordingly cellular damage decrease.Citation17,Citation18 Carnitine has been shown to have a protecting effect in IR damage developed in kidneys because of clamping of renal arteries.Citation19–22 Carnitine has been effective in prevention of IR damage developed in different organs.Citation23–27 Carnitine also decreases renal damage developed because of several agents.Citation28–32
Although carnitine has been shown to decrease lung damage in infrarenal AIR models, effect of carnitine on renal damage in this model has not been studied.Citation33 This study was designed to investigate the effect of carnitine, an antioxidant agent, in rat models in decreasing IR damage developing in kidneys following infrarenal aortic occlusion–reperfusion and in preventing kidneys from this kind of damage.
SUBJECTS AND METHODS
In this study, 40 male rats of Sprague-Dawley type weighing between 220 and 280 g provided from Animal Laboratory of University of Süleyman Demirel were used. Rats were inhabited in cages in rhythm of 12 h night and 12 h day, ambient temperature of 24–26°C, and humidity of 50–60% before experiment. Standard pellet feeds were used to feed rats and regular tap water was used. Twelve hours before experiment, feeding of rats was stopped, but not water. Care of all rats was done according to Experimental Animal Usage and Principles regulated by National Health and Medical Research Council and according to Guide for Experimental Animal Care and Usage prepared and issued by National Institution of Health (NIH issue no. 85–23, 1985 revised). Study protocols and experimental methods were approved by local Institutional Ethics Committee of Experimental Animals in University of Süleyman Demirel.
Experimental Protocols
Forty male rats of Sprague-Dawley type were evenly (n = 8) randomized to five groups. Duration of IR in rats exposed to IR was applied as 3 h ischemia and then 1 h of reperfusion as in the study of Ozca et al.Citation34 In groups administered carnitine, dosage of carnitine was 40 mg/kg for low-dose group and 200 mg/kg for high-dose group as in the study of Boonsanit et al.Citation35
Group I (SHAM, n = 8): Laparotomy and infrarenal AA dissection were applied with same surgical duration and stress as in other groups but no occlusion was applied to infrarenal AA.
Group II (CC, n = 8): Laparotomy and infrarenal AA dissection were applied but no occlusion was applied to infrarenal AA. About 200 mg/kg of carnitine was administered i.p. 15 min before laparotomy (carnitene vial 1 g–5mL, Santa Farma).
Group III (AIR, n = 8): About 3 mL of saline was administered i.p. 15 min before occlusion. After laparotomy and infrarenal AA dissection, infrarenal AA was clamped by atraumatic microvascular clamp for 180 min and then was exposed to 60 min of reperfusion.
Group IV (AIR+LDC, n = 8): About 40 mg/kg of carnitine was administered i.p. 15 min before infrarenal AA dissection and occlusion and then 180 min of ischemia and then 60 min of reperfusion was applied.
Group V (AIR+HDC, n = 8): About 200 mg/kg of carnitine was administered i.p. 15 min before infrarenal AA dissection and occlusion and then 180 min of ischemia and then 60 min of reperfusion was applied.
Aortic Ischemia–Reperfusion
Anesthesia was induced by intramuscular injection of 50 mg/kg of ketamine hydrochloride (Ketalar® flacon, Parke-Davis, USA) at the beginning of experiment. Process was performed on supine rats under a warming lamp. Midline laparotomy was applied to rats after their skins were treated aseptically and then 10 mL of saline solution was administered i.p. to preserve fluid balance. After bowels were moved away by wet surgical gauze, infrarenal AA were carefully explored. Infrarenal AA were clamped by atraumatic microvascular clamp (vascu-statts II, midi straight 1001-532; Scanlan Int., St. Paul, MN, USA) and abdominal incision was temporarily covered up by a plastic clothing to minimize loss of heat and fluid. After 180 min, abdomen was explored again and clamp on infrarenal AA was removed and 60 min of reperfusion was applied. Aortic ischemia was validated by loss of pulsation on distal aorta, and aortic reperfusion was validated by return of pulsation on distal aorta after removal of clamp. Thereby no-reflow phenomenon was excluded. After duration of reperfusion all rats were killed by decapitation.
Sampling of Tissue and Blood
Before rats were killed, 3 mL of blood samples were collected from vena cava inferior for biochemical examinations. Blood samples were centrifuged and serums were separated on the same day and kept at −80°C for biochemical examinations. After rats were sacrificed, both kidneys were removed from abdomen for enzymatic and histopathological examinations. Right kidneys were kept at −80°C consecutively for biochemical examinations. Left kidneys were kept for histopathological examinations.
Biochemical Measurements
Serum blood urea nitrogen (BUN) and creatinine measurements were done by spectrophotometric methods using appropriate commercial kits by Abbott Aeroset Instrument. Frozen right kidneys allocated for biochemical examinations were weighed and were homogenized in ice bath in 100 mmol/L of phosphate buffer (pH 7.4) containing 0.05% sodium azide (Ultra Turrax T25, Janke & Kunkel GmbH & Co. KG, Staufen, Germany). Homogenate was sonicated for 30 s (Sonoplus UW 2070, Bandelin, Berlin, Germany) and then centrifuged (+4°C, 5000g for 15 min). In resultant supernatants, malondialdehyde (MDA) levels and enzymatic activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) were measured. Protein content of tissue was determined by Lowry method.Citation36
Malondialdehyde Measurement
At the end of reperfusion process MDA levels, a marker of lipid peroxidation, were determined by double heating method of Draper and Hadley.Citation37 The principle of the method was spectrophotometric measurement of color developing during reaction of MDA with thiobarbituric acid. For this purpose, 2.5 mL of 100 g/L trichloroacetic acid was added to 0.5 mL of supernatant in each centrifuge tube. Tubes were cooled down under tap water and then centrifuged for 10 min at 3000g. For each test tube, 2 mL of supernatant was added to 1 mL of 6.7 g/L thiobarbituric acid and the tubes were put into boiled water bath for 15 min. Solution was cooled down under tap water and its absorbance was measured by using a spectrophotometer of 532 nm (Shimadzu UV-1601). MDA standard solution was prepared by using tetraethoxypropane. Solutions with different concentrations were prepared by serial dilutions. Sample concentrations were calculated by drawing standard absorbance graphic and expressed as millimole per gram tissue protein (mmol/g protein).
Measurement of Superoxide Dismutase Activity
SOD activity was measured using methods of Spitz and OberleyCitation38 and Woolliams.Citation39 Measurement of SOD activity were done based on xanthine oxidase reaction producing superoxide radicals forming a red formazan dye reacting with 2-(4-iodophenol)-3-4(4-nitrophenol)-5-pheniltetrazoliumchloride. SOD activity was determined as degree of inhibition of this reaction. Results were expressed as units per milligram of tissue protein (U/mg protein).
Measurement of Catalase Activity
CAT activity was measured according to Aebi's method.Citation40 This method is based on the principle of determining the rate constant of fractioning rate of hydrogen peroxide (H2O2). Rate constant was calculated using the formula k = (2.3/–t)(a/b) log (A1/A2). In this formula, A1 and A2 are absorbance values at 0 s and 15 s, respectively, and a is the dilution factor and b is the protein content of supernatant. Results were expressed as katal per gram tissue protein (k/g protein).
Measurement of Glutathione Peroxidase
Glutathione peroxidase (GPx) activity was studied according to method of Paglia and Valentine.Citation41 In the presence of hydrogen peroxidase, GPx catalyzes oxidation of reduced glutathione to oxidized glutathione. In the presence of hydrogen peroxide, oxidized glutathione produced by GPx is reduced to glutathione by contribution of glutathione reductase and NADPH. GPx was calculated by measuring the decrease of absorbance at 340 nm during oxidation of NADPH to NADP+ and expressed as units per gram tissue protein (U/gram protein).
Histological Examination
Histological examination of renal tissues and calculation of scores of damage were done by the method of Sahin et al.Citation42 Tissue samples were fixed for 24 h in buffered neutral formaldehyde solution (10%). All of the samples were routinely observed in tissue observation device and paraffin blocks were prepared. Serial cross-sections of 4 µm for each tissue sample by microtome from these paraffin blocks were obtained and these paraffin cross-sections were dyed with hematoxylin-eosin. Histopathological examination was done with light microscopy (NIcon microscope ECLIPSE E600W, Tokyo, Japan) and cross-sections were photographed by digital camera (Microscope Digital Camera DP70, Tokyo, Japan). All renal tissues were examined for tubular necrosis atrophy, tubular degeneration, glomerular damage, vascular congestion thrombosis, and interstitial inflammation parameters by the same pathologist blindly. Level of changes in each cross-section was graded from 0 to +3 according to intensity and penetration of changes. After this process, values of intensity and penetration were combined and values calculated for each cross-section were summed and results were defined as 0 (no findings), +1 (very low), +2 (low), +3 (medium), +4 (intensive), +5 (severe), and +6 (very severe). Scores of each group were summed and total score of histological damage (TSHD) were calculated for each group.
Statistical Analyses
Analyses of study data were performed using SPSS 10.0. Data following normal distribution were expressed as mean ± standard deviation and data not following normal distribution were expressed as median. During statistical evaluation of digital data following normal distribution (SOD, GPx, and CAT) single-variant analyses (ANOVA) were used for analyzing differences between groups and then Turkey's honestly significant difference test was used; p <0.05 was defined as statistically significant. During statistical analyses of digital data not following normal distribution (BUN, creatinine, MDA, and TSHD) and histological findings (degrees of tubular necrosis atrophy, tubular degeneration, glomerular damage, vascular congestion thrombosis, and interstitial inflammation), statistically significant differences between groups were determined by Kruskal–Wallis test and differences between two groups were determined by Mann–Whitney U-test. p <0.05 was defined as statistically significant. Relations between biochemical data (BUN, creatinine, MDA, SOD, GPx, and CAT) and relations of biochemical data with TSHD were evaluated by Pearson correlation test.
RESULTS
Biochemical Measurements
Results of BUN (mg/dL), creatinine (mg/dL) in serum samples and results of MDA (mmol/g protein), SOD (U/mg protein), GPx (U/g protein), and CAT (k/g protein) in renal tissue samples are shown in . Plasma creatinine level in AIR group was significantly higher than other groups (p < 0.01). However, there was no significant difference between AIR+LDC group and AIR+HDC group (p > 0.05). Tissue MDA levels of AIR group were significantly higher than SHAM, CC, and AIR+HDC groups (p < 0.01). There was no statistically significant difference between AIR group and AIR+LDC group (p >0.05). Activity of SOD in renal tissue was higher in AIR group than SHAM group (p < 0.05). For activities of other antioxidant enzymes, GPx and CAT, there were no significant differences between groups (p >0.05).
Table 1. Biochemical measurements of groups.
Histological Examinations
Comparative results of types of histological damages and TSHD according to groups are shown in and microscopic photographs of renal tissue samples of groups are shown in . When we compared groups for types of histological damage, median values of tubular necrosis atrophy and tubular degeneration in AIR+LDC group (p =0.001) and median values of tubular necrosis atrophy, tubular degeneration, glomerular damage, and vascular congestion thrombosis in AIR+HDC group (p = 0.001, p =0.001, p =0.004, and p = 0.001, respectively) were significantly lower than AIR group. When we compared AIR+LDC group with AIR+HDC group, median values of glomerular damage and vascular congestion thrombosis were significantly lower in AIR+HDC group (p =0.008). However, for median values of interstitial inflammation which were significantly higher in AIR group than in SHAM and CC groups, no significant changes were observed between low-dose and high-dose carnitine groups (p > 0.05).
Figure 1. Comparison of types of histological damage and total score of histological damage according to groups. Kruskal–Wallis test for comparison and Mann–Whitney U-test for determining difference between two groups were used.
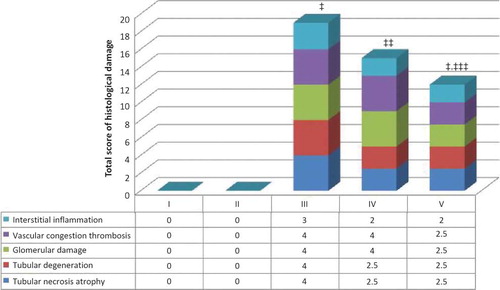
Figure 2. Renal tissue samples of Group I (SHAM) and Group II (CC). Both showed similar morphological characteristics (H&E, ×100).
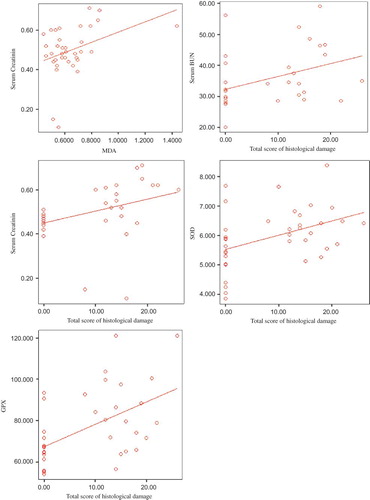
Figure 3. Renal tissue samples of Group III (AIR). C, congestion; black arrow, necrosis; black star, degenerative changes (hydrophic and vacuolar); ii, interstitial inflammation (H&E, ×100, 200, 200, 400).
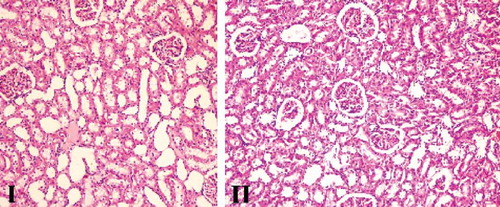
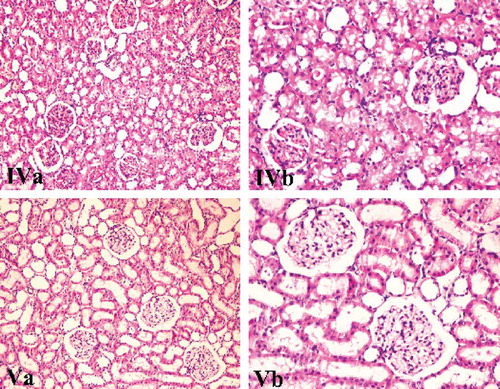
Figure 4. Renal tissue samples of Group IV (AIR+LDC) and Group V (AIR+HDC). Congestion, necrosis, degenerative changes (hydrophic and vacuolar), and interstitial inflammation were mild-medium level, and there was a significant decrease in AIR+HDC group (H&E, ×100, 200).
When groups were evaluated for TSHD, it was found to be significantly higher in SHAM and CC groups than in AIR group (p < 0.001) and was significantly lower in AIR+LDC and AIR+HDC groups than AIR group (p =0.001 and p <0.001, respectively). To understand if carnitine effect is dose related or not, we compared AIR+LDC group and AIR+HDC group and we found TSHD was significantly lower in AIR+HDC group (p = 0.013).
Correlations
When we have studied relation between biochemical parameters and relation between biochemical parameters and TSHD, a positive but weak correlation was determined between serum creatinine and MDA level (r = 0.14, p = 0.026). A positive correlation was determined between TSHD and serum BUN (r = 0.15, p =0.028), serum creatinine (r = 0.14, p = 0.024), level of tissue SOD enzyme (r = 0.18, p =0.01), and level of tissue GPx enzyme (r = 0.27, p = 0.001) ().
Figure 5. The relationship between serum creatinine and tissue MDA levels (r = 0.14, p = 0.026), total score of histological damage and serum BUN (r = 0.15, p = 0.028), serum creatinine (r = 0.14, p = 0.024), tissue SOD (r = 0.18, p = 0.01), and tissue GPx (r = 0.27, p = 0.001). Pearson correlation test was used.
DISCUSSION
In this study designed to examine the role of carnitine in renal damage developed after infrarenal aortic occlusion and reperfusion, carnitine was shown to partially decrease IR damage in kidneys. Carnitine decreased damage related to AIR in both tissue and blood levels. Histopathological changes and plasma creatinine and tissue MDA levels found to be higher in AIR group showed a dose-related decrease by carnitine administration. In our study, we used MDA, BUN, and creatinine measurements as biochemical markers for evaluation of IR damage. Lipid peroxidation developed by free oxygen radicals released after IR damage is believed to have important destructive effects on cellular membrane. Lipid peroxidation causes changes in viscosity and permeability of cellular membrane. As a result membrane proteins are destroyed and this process as a whole is accepted to be one of the important factors leading to cellular death following reperfusion damage.Citation43 As an end product of lipid peroxidation in tissue and plasma, MDA is currently used as a marker of IR damage. In many studies serum and/or tissue MDA levels were shown as increased.Citation19,Citation20,Citation22,Citation44–47 In our study, serum creatinine and tissue MDA levels were significantly higher in AIR group than other groups. Also there was a positive correlation between serum creatinine and tissue MDA levels and between creatinine values and TSHD. This also shows that we successfully developed IR damage in our study model.
In this study, serum creatinine levels were high in AIR group but in groups administered low and high dose of carnitine they were significantly lower; however, there were no significant differences between low- and high-dose groups. MDA level was high in AIR group but it was lower in both low-dose (40 mg/kg) and high-dose (200 mg/kg) carnitine groups; however, statistically significant difference was observed in high-dose carnitine group. Carnitine was shown to provide significant decreases in tissue MDA levels in studies of Ergun et al.Citation19 in rabbits and Gorur et al.Citation22 in rats investigating protective effects of carnitine in renal damage related to IR developed by clamping renal arteries. Mister et al. showed in their studies with the same method that carnitine protected phospholipids and cellular membrane integrity by preventing lipid peroxidation and release of free oxygen radicals. Mister et al.Citation20 also showed that carnitine prevented increase in MDA levels in kidneys after reperfusion. Besides, carnitine also prevents increase in MDA levels in IR damage in several organs and in renal damage caused by several agents.Citation23,Citation24,Citation28,Citation29,Citation32 According to all these findings, it is possible to say carnitine prevents lipid peroxidation in infrarenal AIR models in dose-related fashion and this effect of carnitine also contributes in the prevention of possible renal damage.
In our study, we measured activities of SOD, GPx, and CAT enzymes in renal tissue to evaluate antioxidant activity of carnitine. SOD, CAT and GPx are antioxidant enzymes with complementary characteristics for each other. These antioxidant enzymes step in as a compensatory mechanism when oxidative stress increases. A reaction catalyzed by an enzyme which activity and/or quantity is decreased, can be catalyzed by another enzyme or harmful substrate can be directed to a different path.
Our findings show that level of SOD enzyme is significantly higher in AIR group than control group. Also there is a positive correlation between SOD and GPx enzyme and TSHD. There is no significant difference between groups for levels of GPx and CAT enzymes. In studies, where protective activities of gadolinium chloride, aprotinin, and erythropoietin were investigated in infrarenal AIR model, SOD was determined as increased in IR period.Citation47–49 Baker et al. determined that SOD showed a significant increase in renal tissue in IR period in IR damage model developed by clamping of renal arteries. In the same study, IR damage was found to decrease significantly by administration of antioxidant treatment with SOD enzyme before reperfusion in IR group.Citation42 Moreover, Hoch et al.Citation50 found in their study of lower-extremity IR model in dogs that mannitol and SOD enzyme have a neuromuscular protective effect. Also in IR of small intestine and IR of liver, SOD enzyme was shown to increase.Citation51,Citation52 On the contrary of our findings, in several studies investigating renal IR damage SOD enzyme was found to be low in IR period.Citation53–56 Different antioxidant enzyme levels in IR period might be related to several factors: extent of IR damage, durations of ischemia and reperfusion, characteristics of damaged organ, and developing form of IR damage. In fact, Dobashi et al. showed that levels of antioxidant enzymes change according to durations of ischemia and reperfusion in IR damage in kidney. With increase in duration of ischemia, level of enzyme decreases and with increase in duration of reperfusion, antioxidant level increases.Citation57
In our study, activity of SOD enzyme was found as increased with AIR but activities of GPx and CAT enzymes showed no changes. Increase in activity of SOD enzyme with AIR was thought to be related to compensation of free oxygen radicals increased because of AIR. However, as there was no increase in activities of GPx and CAT enzymes in AIR group, hydrogen peroxide (H2O2−) formed from superoxide radicals (O2−) with increased SOD activity might not be sufficiently destroyed and might be causing lipid peroxidation in cellular membrane transforming into hydroxyl radical (HO·) by Fenton reaction. As a marker of this process, we might be finding high tissue MDA levels in AIR group. Administration of carnitine did not change SOD enzyme activity and there was no difference for GPx and CAT enzyme activities between the groups. The reason of this may be related with Carnitine's effect of transferring fatty acids to mitochondria and beta-oxidation in ischemic period, which causes alternative energy sources, rather than its antioxidant effect.
In this study, histopathological findings with light microscopy showing IR damage in AIR group were tubular necrosis atrophy, tubular degeneration, glomerular damage, vascular congestion thrombosis, and interstitial inflammation. Compared with AIR group, median values of tubular necrosis atrophy and tubular degeneration in AIR+LDC group and median values of tubular necrosis atrophy, tubular degeneration, glomerular damage, and vascular congestion thrombosis in AIR+HDC group were significantly lower. When AIR+LDC group is compared with AIR+HDC group, median values of glomerular damage and vascular congestion thrombosis were significantly lower in AIR+HDC group. When groups are evaluated for TSHD, it was found to be significantly higher in AIR group than SHAM and CC groups but lower in AIR+LDC and AIR+HDC groups than AIR group. To understand if the effect of carnitine is dose related or not, we compared AIR+LDC group and AIR+HDC group and found TSHD was significantly lower in AIR+HDC group. Therefore possible histopathological changes after infrarenal aortic occlusion and reperfusion decreased in a dose-related fashion by carnitine administration. Ergun et al.Citation19 in rabbits and Onal et al.Citation21 in rats showed that in IR damage developed by clamping of renal arteries carnitine decreased histopathological changes in kidneys. In similar models, Mister et al. showed that carnitine improved tubular lesions developed by renal IR damage in their studies on rat. Carnitine was also shown to prevent histopathological changes in IR damage of other organs like medulla spinalis and testis in animal models.Citation23,Citation24 Carnitine was shown to prevent histopathological changes in renal damage related to gentamicin in the study of Kopple et al.;Citation30 in myoglobulinuric acute renal failure in the study of Aydogdu et al.;Citation28 in chronic cyclosporine A nephrotoxicity in the study of Origlia et al.;Citation31 in renal damage related to methotrexate in the study of Sener et al.;Citation32 in cisplatin nephrotoxicity in the study of Aleisa et al.Citation29 According to our findings and all these results, carnitine was shown to have a protective effect in renal damage developed by IR and also shown to be effective in preventing renal damage developing after infrarenal AIR. Although precise mechanism of this beneficial effect of carnitine preventing IR damage is not known, carnitine might be increasing ATP production by inducing mitochondrial energy production, transfer of fatty acids to mitochondria, and oxidation slowed down in ischemic process and consequently alternative energy sources might be used in IR period to protect cellular membrane integrity and to decrease cellular damage.
As a conclusion, in our study carnitine was shown to prevent renal damage in infrarenal AIR model of rats. This result may open new prospects to us in the prevention of renal IR damage during surgery of aorta.
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Parrino PE, Laubach VE, Gaughen JR, Jr, Inhibition of inducible nitric oxide synthase after myocardial ischemia increases coronary flow. Ann Thorac Surg. 1998;66:733–739.
- Koksel O, Ozdulger A, Aytacoglu B, The influence of iloprost on acute lung injury induced by hind limb ischemia-reperfusion in rats. Pulm Pharmacol Ther. 2005;18:235–241.
- Haimovici H. Muscular, renal, and metabolic complications of acute arterial occlusions: Myonephropathic-metabolic syndrome. Surgery. 1979;85:461–468.
- Ellenberger C, Schweizer A, Diaper J, Incidence, risk factors, and prognosis of changes in serum creatinine early after aortic abdominal surgery. Intensive Care Med. 2006; 32:1808–1816.
- Tallgren M, Niemi T, Pöyhiä R, Acute renal injury and dysfunction following elective abdominal aortic surgery. Eur J Vasc Endovasc Surg. 2007;33:550–555.
- Braams R, Vossen V, Lisman BA, Outcome in patients requiring renal replacement therapy after surgery for ruptured and nonruptured aneurysm of the abdominal aorta. Eur J Vasc Endovasc Surg. 1999;18:323–327.
- Klausner JM, Anner H, Paterson IS, Lower torso ischemia-induced lung injury is leukocyte dependent. Ann Surg. 1988;208:761–767.
- Yakut N, Yasa H, Lafci BB, The influence of levosimendan and iloprost on renal ischemia-reperfusion: An experimental study. Interact Cardiovasc Thorac Surg. 2008;7:235–239.
- Kiris I, Okutan H, Savas C, Gadolinium chloride attenuates aortic occlusion-reperfusion-induced myocardial injury in rats. Saudi Med J. 2007;28:347–352.
- Gamulin Z, Forster A, Morel D, Effects of infrarenal aortic cross-clamping on renal hemodynamics in humans. Anesthesiology. 1984;61:394–399.
- Gamulin Z, Forster A, Simonet F, Aymon E, Favre H. Effects of renal sympathetic blockade on renal hemodynamics in patients undergoing major aortic abdominal surgery. Anesthesiology. 1986;65:688–692.
- Welch M, Knight DG, Carr HM, Smyth JV, Walker MG. Influence of renal artery blood flow on renal function during aortic surgery. Surgery. 1994;115:46–51.
- Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Thoracoabdominal aortic aneurysms associated with celiac, superior mesenteric, and renal artery occlusive disease: Methods and analysis of results in 271 patients. J Vasc Surg. 1992;16:378–389, discussion 389–390.
- Kucuker SA, Astan A, Tasdemir O. Postoperative complications of thoracic aortic aneurysm surgery. Turkiye Klinikleri J Surg Med Sci. 2005;1:99–104.
- Fabriello RG, Ferrano TG, Golden GT, DeMattei M. Systemic acetyl-L-carnitine elevates nigral levels of glutathione and GABA. Life Sci. 1998;43:289–292.
- Fabriello RG, Calabrese F. Prevention of ischemia induced increase in MDA by acetyl carnitine. Ann Neurol. 1988; 24:114–118.
- Hoppel C. The physiological role of carnitine. In: Ferrari R, DiMauro S, Sherwood G, eds. L-Carnitine and Its Role in Medicine: From Function to Therapy. London: Academic Press; 1992:5–17.
- Matsumura M, Hatakeyama S, Koni I, Mabuchi H. Effect of L-carnitine and palmitoyl- L-carnitine on erythroid colony formation in fetal mouse liver cell culture. Am J Nephrol. 1998; 18:355–358.
- Ergun O, Ulman C, Kilicalp AS, Ulman I. Carnitine as a preventive agent in experimental renal ischemia – Reperfusion injury. Urol Res. 2001;29:186–189.
- Mister M, Noris M, Szymczuk J, Propionyl-L-carnitine prevents renal function deterioration due to ischemia/reperfusion. Kidney Int. 2002;61:1064–1078.
- Onal A, Astarcioglu H, Ormen M, Atila K, Sarioglu S. The beneficial effect of L-carnitine in rat renal ischemia-reperfusion injury. Ulus Travma Acil Cerrahi Derg. 2004;10:160–167.
- Gorur S, Bagdatoglu OT, Polat G. Protective effect of -carnitine on renal ischemia – Reperfusion injury in the rat. Cell Biochem Funct. 2005;23:151–155.
- Dokmeci D, Inan M, Basaran U, Protective effect of L-carnitine on testicular ischemia – Reperfusion injury in rats. Cell Biochem Funct. 2007;25:611–618.
- Rahman A, Ustündag B, Burma O, Ozercan IH, Erol FS. Neuroprotective effect of regional carnitine on spinal cord ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2001; 20:65–70.
- Di Giacomo C, Latteri F, Fichera C, Effect of acetyl-L-carnitine on lipid peroxidation and xanthine oxidase activity in rat skeletal muscle. Neurochem Res. 1993; 18:1157–1162.
- Reznick AZ, Kagan VE, Ramsey R, Antiradical effects in L-propionyl carnitine protection of the heart against ischemia-reperfusion injury: The possible role of iron chelation. Arch Biochem Biophys. 1992;296:394–401.
- Akisu M, Kultursay N, Coker I, Huseyinov A. The effect of L-carnitine on platelet activating factor concentration in the immature rat model of hypoxic-ischemic brain injury. Acta Med Okayama. 1998;52:183–187.
- Aydogdu N, Atmaca G, Yalcin O, Taskiran R, Tastekin E, Kaymak K. Protective effects of L-carnitine on myoglobinuric acute renal failure in rats. Clin Exp Pharmacol Physiol. 2006;33:119–124.
- Aleisa AM, Al-Majed AA, Al-Yahya AA, Reversal of cisplatin-induced carnitine deficiency and energy starvation by propionyl-L-carnitine in rat kidney tissues. Clin Exp Pharmacol Physiol. 2007;34:1252–1259.
- Kopple JD, Ding H, Letoha A, L-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol Dial Transplant. 2002;17:2122–2131.
- Origlia N, Migliori M, Panichi V, Protective effect of L-propionylcarnitine in chronic cyclosporine-a induced nephrotoxicity. Biomed Pharmacother. 2006;60:70–81.
- Sener G, Eksioglu-Demiralp E, Cetiner M, L-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol. 2006;22:47–60.
- Berkan O, Yildiz E, Guneç F, Katrancioglu N, Günay I, Dogan K. The effect of pentoxifylline, carnitine and ascorbic acid on improvement of lung injury caused by ischemia-reperfusion. Turkish J Thorac Cardiovasc Surg. 2002;10:92–95.
- Ozca AV, Sacar M, Aybek H. The effects of iloprost and vitamin C on kidney as a remote organ after ischemia/reperfusion of lower extremities. J Surg Res. 2007;140:20–26.
- Boonsanit D, Kanchanapangka S, Buranakarl C. L-carnitine ameliorates doxorubicin- induced nephrotic syndrome in rats. Nephrology (Carlton). 2006;11:313–320.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431.
- Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18.
- Woolliams JA, Wiener G, Anderson PH, McMurray CH. Variation in the activities of glutathione peroxide and super oxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci. 1983;34:253–256.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Sahin O, Sulak O, Yavuz Y, Lithium-induced lung toxicity in rats: The effect of caffeic acid phenethyl ester (CAPE). Pathology. 2006;38:58–62.
- Garcia JJ, Reiter RJ, Guerrero JM, Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 1997;408:297–300.
- Baker GL, Corry RJ, Autor AP. Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion. Protective effect of superoxide dismutase. Ann Surg. 1985;202:628–641.
- Ancioglu A, Aydin S, Turkozkan N, Durmus O. The effect of allopurinol on Na+K+ATPase related lipid peroxidation in ischemic and reperfused rabbit kidney. Gen Pharmacol. 1994;25:341–344.
- Selcuk NY, Yakan B, San A. The evaluation of lipid peroxidation and alpha-tocopherol treatment in experimental warm renal ischemia and reperfusion. Turk Nefroloji Diyaliz ve Transplantasyon Dergisi. 1996;1:5–10.
- Kiris I, Okutan H, Savas C, Yonden Z, Delibas N. The effect of gadolinium chloride on renal injury in the model of experimental aortic ischemia-reperfusion. Turkish J Vasc Surg. 2005;14:13–18.
- Karabiga M, Kiris I, Yilmaz N, Altuntas İ, Karahan N, Okutan H. The effect of aprotinin on renal injury in an experimental model of aortic ischemia-reperfusion. Turkish J Vasc Surg. 2007;16:9–18.
- Kiris I, Kapan S, Kilbas A, The protective effect of erythropoietin on renal injury induced by abdominal aortic-ischemia-reperfusion in rats. J Surg Res. 2008; 149:206–213.
- Hoch JR, Stevens RP, Keller MP, Silver D. Recovery of neuromuscular function during reperfusion of the ischemic extremity: Effect of mannitol and superoxide dismutase. Surgery. 1991;110:656–662, discussion 662–663.
- Topcu I, Vatansever S, Var A, Cavus Z, Cilaker S, Sakarya M. The effect of misoprostol, a prostaglandin E1 analog, on apoptosis in ischemia-reperfusion induced intestinal injury. Acta Histochem. 2007;109:322–329.
- Chen CF, Hsueh CW, Tang TS, Wang D, Shen CY, Pei JS. Reperfusion liver injury induced superoxide dismutase and catalase expressions and the protective effects of N-acetyl cysteine. Transplant Proc. 2007;39:858–860.
- Unlu A, Sucu N, Tamer L, Effects of daflon on oxidative stress induced by hind limb ischemia/reperfusion. Pharmacol Res. 2003;48:11–15.
- Ozan E, Koyuturk L, Sapmaz T. An investigation on the effects of prostaglandin E1 as antioxidant in experimental renal ischemia reperfusion injury. Firat Med J. 2004;9:67–77.
- Aydogdu N, Kaymak K, Yalcin O. Effects of N-acetylcysteine in renal ischemia/reperfusion injury in the rats. Firat Med J. 2005;10:151–155.
- Aktoz T, Aydogdu N, Alagol B, Yalcin O, Huseyinova G, Atakan IH. The protective effects of melatonin and vitamin E against renal ischemia-reperfusion injury in rats. Ren Fail. 2007;29:535–542.
- Dobashi K, Ghosh B, Orak JK, Singh I, Singh AK. Kidney ischemia-reperfusion: Modulation of antioxidant defenses. Mol Cell Biochem. 2000;205:1.