Abstract
Aim: To investigate the effect of exogenous normal lymph on kidney injury in disseminated intravascular coagulation (DIC) rats and to probe its mechanism. Methods: The DIC model was established by intravenous injection of Dextran 500. After 6 min, normal lymph without cell components was infused in the lymph group. After 40 min, the renal and coagulation function indices and renal histomorphology were observed. Results: Serum urea and creatinine in the model group were significantly higher than in the control and lymph groups. Renal morphological study showed red blood cell silting and casts forming in the model group. The prothrombin time (PT), prothrombin time ratio (PTR), activated partial thromboplastin time (APTT), and thrombin time of lymph and model groups were higher than those in the control group, whereas fibrinogen was lower. The PT, PTR, and APTT were prolonged in the lymph group than in the model group. The platelet functions of the lymph and model groups were higher than in the control group, but platelet aggregation rate and thrombosis-forming indices were lower than in the control group; the platelet adhesive and aggregation rates and thrombosis dry weight of the lymph group were lower than those of the model group. Conclusion: Exogenous normal lymph could alleviate kidney injury in DIC rats, which may be related to the improving coagulation function.
INTRODUCTION
Microcirculatory disturbance and abnormal hemorheology with the ensuing coagulation dysfunction exacerbate the deterioration of primary disease states has been documented in the process of many diseases. Disseminated intravascular coagulation (DIC) is a pathologic process in which a widespread systemic activation of coagulation leads to microvascular thrombosis. This disseminated thrombus formation has been proposed as a major contributor to the development of multiple organ dysfunction syndrome (MODS).Citation1–3 Studies from our laboratory suggested that exogenous normal lymph has a beneficial therapeutic effect on rats subjected to severe hemorrhagic shock; its effect is significantly better than that by the same amount of normal saline as well as albumin solution;Citation4,5 and the normal lymph from healthy dog has a similar effect on endotoxic shock than plasma.Citation6 The anti-shock effect of lymph is difficult to explain by restoration of blood volume or colloid osmotic pressure. Moreover, it has been reported that normal lymph can alleviate the mesenteric microcirculatory disturbance in rats with DIC induced by Dextran 500Citation7 and endotoxic shock.Citation8 The aim of this study was to investigate the effects of exogenous normal lymph on renal function and morphology in a rat model of DIC induced by Dextran 500 and to probe its mechanism focusing on changes in the coagulation function, based on the theory that microthrombus formation is the major mechanism of kidney injury during the process of DIC.
METHODS
Animals and Grouping
Thirty healthy specific pathogen free (SPF) Wistar rats (supplied by the Chinese Academy of Medical Sciences Animal Breeding Center) weighing 240–300 g were used in this study. Animals were maintained under barrier-sustained conditions with 12:12 h light–dark cycles and free access to standard laboratory food and water. The research protocol that was carried out after a minimal acclimation period of 5 days complied with the “Guide for the Care and Use of Laboratory Animals” and was approved by the Institutional Animal Use and Care Committee of the Medical School, Hebei North University. Rats were fasted for 12 h before the experiment, but allowed free access to water. Rats were divided equally into three groups: control group, DIC model group (model group), and DIC lymph-treated group (lymph group). The DIC model was established by injection of Dextran 500 in model and lymph groups.
Extraction and Preparation of Exogenous Normal Lymph
After anesthetization by 25 mg/kg pentobarbital sodium intravenously, a midline laparotomy was performed on healthy beagle dogs and their mesenteric lymph was collected continuously into heparin-wetted sterile test tubes. Briefly, the bowel was eviscerated and rotated to the left. The mesenteric duct and accessory lymphatic (located adjacent to the superior mesenteric artery) were then isolated from the surrounding structures using blunt dissection. The intestinal lymphatic duct was cannulated with a self-made metal lymphatic catheter. Lymph samples were centrifuged for 15 min at 315 × g to remove all cellular components and stored at −80°C until the test.Citation4,5
DIC Model Duplication and Intervention
All rats were anesthetized by intramuscular injection of 50 mg/kg pentobarbital sodium (2%) and placed in a supine position. The left jugular vein and the right carotid artery were aseptically separated from the surrounding tissues and cannulated using a microcatheter for transfusion and administration. After the rats were heparinized intravenously and stabilized for 15 min, 10% Dextran 500 (10 mL/kg, Pharmacia Biotech, Uppsala, Sweden) was injected through the left jugular vein over 3 min using an infusion pump (ZCZ-50, Zhejiang University Medical Ltd., Hangzhou, China) through which the DIC model was established in the model and lymph groups.Citation7,9 After 6 min, rats of the lymph group were administrated with exogenous normal lymph [the volume of lymph to be infused is 1/15 of the whole blood volume, which (mL) is calculated according to 1/13 of the body weight (g), and the normal lymph is diluted with equal normal saline before using]. The rats of the model group only received an injection of the same amount of normal saline. The transfusion was carried out at a speed of 0.5 mL/min for 9–12 min. Dextran 500 and lymph were replaced by normal saline in the control group.
Detecting Renal Function and Morphology
A blood sample of 7 mL was withdrawn from the carotid artery at the end of 40-min intervention with exogenous normal lymph. The serum was prepared by centrifugation at 850 × g for 10 min using 2 mL blood sample, and the remaining blood sample was used to measure the coagulation function, platelet function, and thrombosis in vitro.
Urea and creatinine contents in serum were determined by an automatic biochemical analyzer (Aeroset, Chicago, IL, USA), and the kit was purchased from Randox Laboratories Ltd. (Shanghai, China). Simultaneously, the renal tissue was harvested in a fixed position and then fixed in 10% neutral buffered formalin. After alcohol gradient dehydration and paraffin embedding, the renal tissue was sectioned and stained with hematoxylin and eosin. Morphological alterations were examined by light microscopy (BH-2, Olympus, Tokyo, Japan) and pictures were taken using digital camera (4500, Nikon, Tokyo, Japan) from 10 randomly chosen areas per sample. All morphological examinations were done by a chief physician of the pathology department of Hebei North University First Hospital without prior knowledge of experimental conditions.
Detecting Coagulation Function
Coagulation indices such as prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (Fib), prothrombin time ratio (PTR), and thrombin time (TT) were determined from an automaticity coagulation analyzer (CA1500, Sysmex, Tokyo, Japan) using 0.9 mL blood sample after anticoagulation by 3.8% citrate.
Detecting the Platelet Function
After transfusion for 40 min, blood sample was collected from the common carotid artery by bleeding and heparinized for further assay. The arterial blood, 3 mL, was used to examine platelet adhesivity and aggregation function. Briefly, after centrifugation at 90 × g for 10 min, the total volume of platelet-rich plasma (1 mL) was prepared. To prepare platelet-poor plasma, 0.55 mL platelet-rich plasma was added to the turntable; 0.45 mL platelet-rich or platelet-poor plasma was added to each of the two colorimetric cups, which were placed into an adhesion instrument for measuring the absorbance value. After operation the rate of platelet adhesion was calculated. The remaining 0.45 mL of platelet-rich plasma was added to the colorimeter cup, mixed with a little stir bar, and embedded in the aggregation instrument. After adding 40–50 μL of aggregation agent adenosine diphosphate (ICN Biomedicals Inc., Costa Mesa, Canada), indices of platelet aggregation were determined using turbidimetric method.
Detecting Thrombosis In Vitro
The whole blood of 0.9 mL without anticoagulation was used to determine thrombosis in vitro using a thrombosis analyzer (3-9D, Maisai Technology and Trade Co., Ltd., Chengdu, China). Length, wet weight, and dry weight of thrombosis were measured and then the thrombosis-forming rate (%) was calculated.
Statistical Analysis
All data were expressed as mean ± SD. The statistical analysis was performed by SPSS software 11.5 (Polar Engineering and Consulting Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used between groups and Student–Newman–Keuls (SNK) test was used within group. A p-value of <0.05 was considered to be statistically significant.
RESULTS
Effect of Exogenous Normal Lymph on Renal Function Indices in DIC Rats
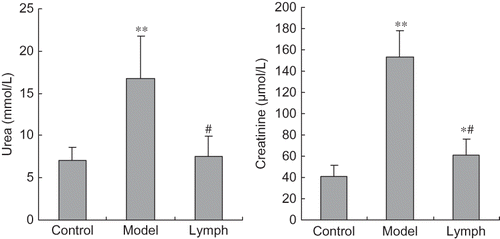
Contents of urea and creatinine in the model group and creatinine in the lymph group were significantly higher than those of the control group (p < 0.01, p < 0.05). Moreover, two indices in the lymph group were lower than those in the model group (p < 0.01, see ).
Effect of Exogenous Normal Lymph on Renal Morphological Change in DIC Rats
A clear glomerular and tubular structure, uniform staining of cytoplasm, and same size nucleus were observed in the kidney of control rats. In the model group, red blood cell (RBC) silting, erythrocyte casts, hemoglobin casts, and protein casts appeared extensively in renal tubules. However, the renal morphological changes were significantly improved by exogenous normal lymph. Glomerular capillaries had a small amount of congestion and interstitial blood vessels were somewhat dilated, as shown in C.
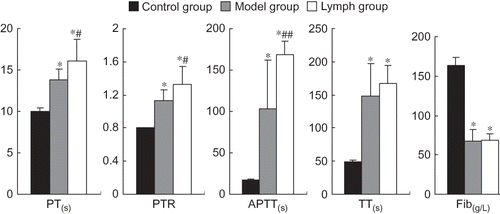
Effect of Exogenous Normal Lymph on Coagulation Function Indices in DIC Rats
The PT, PTR, APTT, and TT of the lymph and model groups were significantly higher than those of the control group, but Fib contents were lower (p < 0.01). The PT, PTR, and APTT of the lymph group were prolonged or increased than those of the model group (p < 0.01, p < 0.05), but Fib contents and TT in both the groups were not statistically different (p > 0.05); see .
Figure 3. Effect of exogenous normal lymph on coagulation function indices in disseminated intravascular coagulation (DIC) rats (mean ± SD, n = 10). PT, prothrombin time; PTR, prothrombin time ratio; APTT, activated partial thromboplastin time; TT, thrombin time; Fib, fibrinogen.
Note: *p < 0.01 versus control group; #p < 0.05, ##p < 0.01 versus model group.
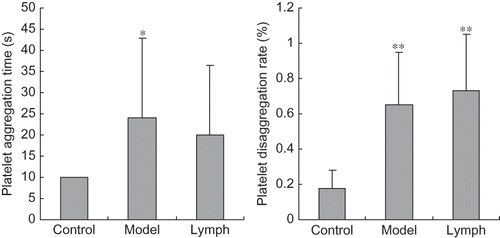
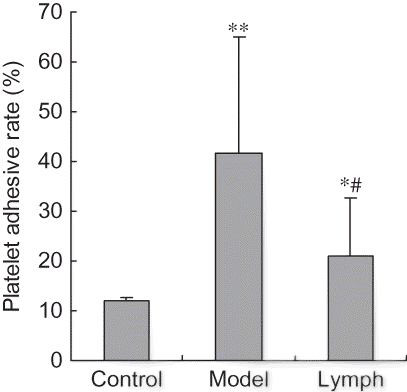
Effect of Exogenous Normal Lymph on Platelet Function Indices in DIC Rats
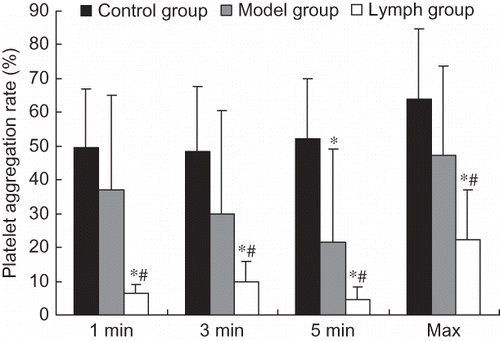
Platelet aggregation rates in the lymph and model groups were significantly lower compared with those of the control group (p < 0.01), and those indices in the lymph group were lower than in the model group (p < 0.01, p < 0.05). The platelet adhesive rate and disaggregation rate of the lymph and model groups were significantly higher than those of the control group (p < 0.01, p < 0.05), and platelet aggregation rate in the lymph group was significantly lower than that of the model group (p < 0.01, see ).
Figure 4. Effect of exogenous normal lymph on platelet aggregation rate in disseminated intravascular coagulation (DIC) rats (mean ± SD, n = 10).
Note: *p < 0.01 versus control group; #p < 0.01 versus model group.
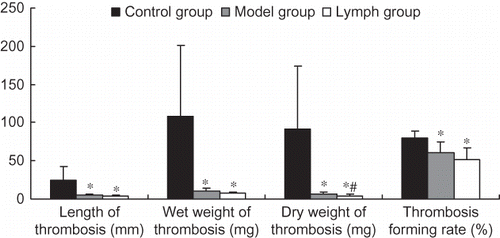
Effect of Exogenous Normal Lymph on Thrombosis In Vitro in DIC Rats
The length, wet weight, and dry weight of thrombosis and thrombosis-forming rate in the lymph and model groups were significantly lower than those of the control group (p < 0.01); however, the dry weight of thrombosis in the lymph group was lower than that of the model group (p < 0.01). Other indices in the two groups were not statistically different (p > 0.05); see .
DISCUSSION
The main reason for microcirculatory disturbance induced by Dextran 500 is related to the intrinsic coagulation pathway activation, thereby forming a mass of microthrombosis.Citation10,11 Diffused microthrombosis may lead to ischemic, hypoxia, and endothelial lesion of involved tissues, such as kidney and lung, which can aggravate coagulation disorder again from the extrinsic coagulation pathway, resulting in microcirculatory disturbance. Therefore, a vicious circle of coagulation disorder (microcirculatory disturbance), that is, organ dysfunction, is aroused, which is presumed to be a pivotal pathogenic factor in the pathogenesis of MODS.Citation12,13 For this reason, the treatment for DIC plays a primary role in alleviating the disturbance of microcirculation, preventing organ injury, as well as correcting the coagulation disorder. Kidney injury is one of multiple organs injury caused by DIC. Microthrombosis formation and microcirculation imbalance induced by coagulation disorders are the main mechanisms. In this study, we examined the effects of normal lymph on kidney injury and coagulation dysfunction in DIC rats. The results confirmed that the exogenous normal lymph can ameliorate renal dysfunction and injury induced by DIC through the mechanism of correcting coagulation dysfunction.
Serum urea and creatinine, biochemical indicators of renal excretion function, caused the renal failure during the process of DIC in rats subjected to Dextran 500 and this was ameliorated by exogenous normal lymph to some extent. From the pathological viewpoint, rats in the model group presented with a large number of casts and exudation in the tubular cavity and these were alleviated by exogenous normal lymph. These results suggest that exogenous normal lymph can relieve kidney injury during the process of DIC induced by Dextran 500.
In rats subjected to Dextran 500, changes in coagulation indicators such as PT, PTR, APTT, TT, and Fib contents indicated the appearance of exhaustive low coagulation state. After intervention by a low dose of exogenous normal lymph, no significant changes between Fib and TT were observed in the model group, suggesting that normal lymph cannot raise the coagulability of DIC rats. The reason may be that the lymph was administrated 6 min after being subjected to Dextran 500 during which time both blood hypercoagulability and exhaustive low coagulation state appears. Moreover, the administration of a low dose of lymph alone does not increase the contents of the clotting factors and platelets. At the same time, it was also observed that values of PT, PTR, and APTT were higher in DIC rats treated by lymph than in rats of the model group. On the basis of the result that no obvious bleeding or errhysis appeared in the abdominal cavity, our study suggested that normal lymph may have a role in reducing coagulation and that the effect is favorable to establish a clotting balance.
Nonetheless, a small amount of exogenous normal lymph reduced the excessive increased platelet adhesion rate and dry weight of thrombus in a relatively short period of time. These results suggest that it is the inhibition of platelet adhesion from which the normal lymph abates clotting function and avoids blood hypercoagulability, thus reducing the consumption of clotting factors and platelets and depressing the microthrombosis. The reduced RBC stasis in renal tissue during histomorphology observation also confirmed this effect. This effect is very beneficial in the reconstruction of the balance among coagulation, anticoagulation, and fibrinolysis in DIC rats induced by Dextran 500. During the experiment, no obvious bleeding or errhysis appeared in the abdominal cavity of rats treated with lymph, which also proved the hypothesis that administration of normal lymph contributes to the reconstruction of the balance between coagulation and anticoagulation effectively.
Moreover, it has been reported that normal mesenteric lymph contains anti-inflammatory properties that decrease the intercellular adhesion molecule-1 expression induced by lipopolysaccharide in pulmonary endothelium.Citation14 The effect might to the mechanism of normal lymph improving the microcirculation disturbance, and improve the oxygen delivery of microvascular endothelial cells, as a result, it was advantaged on coagulation function. So, this effect plays an important role in preserving a stable internal environment and renal function. Nevertheless, whether the lymph achieves this effect through the intervention of fibrinolysis pathway remains to be studied.
It should be pointed out that the main anticoagulant effect of heparin is through enhancing antithrombin III activity and inhibiting the building of thromboplastin, whereas the main mechanism of blood hypercoagulability subjected to Dextran 500 is through activating XII factor and endogenous coagulation pathway. Therefore, the two pathways are different. At the same time, heparin was used in all three groups. Therefore, a small amount of heparin had no effect on platelet and coagulation function.
In this study, normal lymph plasma was obtained from healthy dogs. Is there any immunologic rejection when using heterogeneous animals? In earlier research works of our laboratory, we found that the normal lymph without leukomonocyte from healthy dogs had good intervention roles in hemorrhagic shock and endotoxic shock, and that no immune rejection occurred.Citation3,4,15 In order to observe the possible harmful effect of foreign protein in plasma, the plasma from healthy dog was used in this study as a control. The results showed that there were no microcirculation disorders and immunologic rejection by the foreign protein in plasma from dog. This result confirmed that it was safe to use dogs as donors. Considering the future of plasma applications in medicine, the lymph from healthy dog was used in this research.
Our pilot studyCitation16 found that the apparent viscosity, relative viscosity, lymphocyte aggregation, and lymph plasma viscosity of normal lymph were significantly lower than in plasma, which is beneficial in improving blood microcirculation. The contents of cholesterin and complement in normal lymph were lower than those of plasma, suggesting that normal lymph may have a role in reducing RBC aggregation and inflammatory response. The other components of normal lymph need further examination.
In conclusion, a small amount of exogenous normal lymph from dog may alleviate kidney injury during the process of DIC induced by Dextran 500. The effect may relate to correction of the balance between coagulation and anticoagulation and improvement of the internal environment. It should be noted that the amount of lymph used in this experiment is only 1/15 of the whole blood volume. Although rats were given an equal volume of normal saline in the model group, blood clotting disorders still occur. Therefore, the interventions are difficult to explain by supplementing the blood volume and hemodilution. It should be pointed out that the animal model used in this experiment is acute and the period of observation is short. However, the lasting effects, active substances, and signal pathways that cause lymph to intervene in kidney injury during the process of DIC remain to be clarified.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (30370561, 30770845, and 30971203); Natural Science Foundation of Hebei Province (C2004000649, C2008000503), and a grant from a key scientific and technological project of Hebei Province (02276104D-32, 11276103D-84). No benefits in any form have been received or will be received from a commercial association related directly or indirectly to the subject of this article.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mizock BA. The multiple organ dysfunction syndrome. Dis Mon. 2009;55(8):476–526.
- Vincent JL, De Backer D. Does disseminated intravascular coagulation lead to multiple organ failure? Crit Care Clin. 2005;21(3):469–477.
- Gando S. Microvascular thrombosis and multiple organ dysfunction syndrome. Crit Care Med. 2010;38(Suppl. 2):S35–S42.
- Niu CY, Zhang J, Yao T, . The antagonistic effect of lymph on shock in rat. Lymphology 1998;31 (s):159–162.
- Niu CY, Zhang J, Fan G, . Effect of lymph on mesenteric microcirculation in rat with hemorrhagic shock. Chin J Microcirc. 1996;6(3):1–3.
- Yang YQ, Fan G, Han M, . Effects and mechanisms of lymph on endotoxic shock in rats. Chin J Crit Care Med. 2004;24(9):661–664.
- Han M, Li JJ, Zhang J, . Effect of lymph on acute microcirculatory disturbance of rats induced by dextran 500. Chin J Crit Care Med. 2004;24(9):656–658.
- Zhang LL, Zhang J, Zhao ZG, . Intervention role of lymph plasma on renal and hepatic blood perfusion in rats with endotoxic shock. Chin Crit Care Med. 2010;22(12):740–743.
- Li FL, Liu YK, Niu CY, . Changes in lymph circulation in rats with experimental disseminated intravascular coagulation. Chin Crit Care Med. 2006;18(8):488–490.
- Jastzebski J, Sykes ML, Hilgard P, . Thrombin-induced disseminated intravascular coagulation. III: Modification by dextran infusion. Br J Anaesth. 1975;47(11):1163–1168.
- Moriau M, Rodhain J, Noel H, . Comparative effects of dextrans, gelatin and stable plasma protein solution (SPPS) on the experimental disseminated intravascular coagulation (DIC). Vox Sang. 1974;27(5):411–428.
- Barie PS, Hydo LJ, Pieracci FM, . Multiple organ dysfunction syndrome in critical surgical illness. Surg Infect (Larchmt). 2009;10(5):369–377.
- Niu CY, Li JC, Zhao ZG, . Effect of intestinal lymphatic circulation blockage in two-hit rats. World J Gastroenterol. 2006;12(36):5805–5812.
- Cheng AM, Moore EE, Masuno T, . Normal mesenteric lymph blunts the pulmonary inflammatory response to endotoxin. J Surg Res. 2006;36(2):166–171.
- Zhang J, Niu CY, Zhao ZG. The lymphatic microcirculation & shock. In: Zhao KS and Xu Q, eds. Molecular Mechanism of Severe Shock. Trivandrum: Research Signpost; 2009:131–155.
- Zhao ZG, Niu CY, Hou YL, . Comparison of rheology and ingredient between normal lymph and blood. Chin J Appl Physiol. 2009;25(4):482–484.
![Figure 2. Effect of exogenous normal lymph on renal morphological change in disseminated intravascular coagulation (DIC) rats [hematoxylin and eosin (HE) staining, ×200]. (A) Control group. (B) Model group. (C) Lymph group.](/cms/asset/04a82f59-fd1a-4841-a9d8-0cce8c7f7057/irnf_a_643390_f0002_b.jpg)