Abstract
Background: Anemia in patients with early diabetes mellitus nephrosclerosis (DMN) is more severe than in patients with kidney disease of other origins, and the mechanism for this remains unclear. In this study, we carried out a retrospective study in order to identify the factors associated with anemia in patients with DMN. Methods: To elucidate the factors that influence the severity of anemia in patients with DMN, we carried out a retrospective follow-up study of 124 biopsy-proven DMN cases [mean (SE) age, 55.3 (1.2) years; range, 18–78 years; male/female, 80/44]. First, a cluster analysis was performed using red blood cell counts and hemoglobin (Hb) and hematocrit levels. We then divided the clusters with regard to renal prognosis and survival and carried out simple and multifactorial analysis of clinical data, including the body mass index, age, systolic blood pressure (BP), diastolic BP, duration after the diagnosis of diabetes mellitus, serum albumin levels, blood urea nitrogen (BUN) concentrations, serum creatinine concentrations, the estimated glomerular filtration rate (eGFR) validated in the Japanese population, iron levels, total cholesterol levels, triglyceride levels, fasting blood sugar levels, HbA1c levels, urinary protein secretion, and pathohistological parameters. Results: The factors that were significantly associated with the cluster group that showed severe anemia were sex (p = 0.0162), hypoalbuminemia (p < 0.0001), high BUN concentrations (p = 0.0020), low eGFR (p = 0.0104), and Kimmelstiel–Wilson nodules (p = 0.0022). In addition, hypoalbuminemia (p = 0.0277), high BUN concentrations (p = 0.0338), and a low eGFR (p = 0.0417) were significantly associated with this group in a multifactorial analysis. Conclusion: Our data strongly suggest that hypoalbuminemia is associated with severe anemia in DMN patients.
INTRODUCTION
Diabetes mellitus nephrosclerosis (DMN) is the most common cause of renal failure in industrial countries.Citation1 Renal prognostic factors include anemia,Citation2–4 hyperglycemia,Citation5 hypertension,Citation2,6 a protein-rich diet,Citation7,8 and severe proteinuria.Citation9 Anemia in an early DMN patient is more severe than in patients with kidney disease of other origins.Citation10,11 Although severe anemia in patients with early DMN is a well-known issue, the causes remain unclear.Citation3,12–17 In this study, we investigated the possible factors that are related to the severity of anemia in DMN patients with serum creatinine (SCr) concentrations that were 2 mg/dL or less.
PATIENTS AND METHODS
Subjects
Of the approximately 12,000 serial renal biopsy cases that were examined in the Department of Pathology at the Fukuoka University School of Medicine over a period of 25 years, the cases were not diagnosed with DMN because of SCr < 2 mg/dL, there were 124 cases selected because of that criteria of DMN and SCr < 2 mg/dL. These patients were biopsied because of their characteristics that consisted of a short history of diabetes mellitus (DM), hematuria, nondiabetic retinopathy, rapid progression, and nephritic syndrome. The subjects included 80 males and 44 females whose ages ranged from 18 to 78 years [mean (SE): 55.3 (1.2) years]. The follow-up data were collected by medical chart review as well as by phone or written questionnaires. The mean duration from biopsy to the end of the follow-up period was 6.2 (0.5) years. No subjects received erythropoiesis-stimulating agents (ESAs).
Histopathology of the Kidney
For all renal biopsy specimens, hematoxylin–eosin, periodic acid-Schiff, and periodic acid-methenamine-silver stains were performed. Kimmelstiel–Wilson (KW) nodules or diffuse mesangial proliferation was recognized under polarized light microscopy as hallmarks of DMN for all cases. The evaluation of the glomerular basement membrane thickening (≥400 nM) was performed under an electron microscope. No immunoglobulin deposits were confirmed by the immunofluorescence method. The severity of sclerosis in the glomerulus was evaluated in a blinded manner by a histologic examination of the sectioned kidneys, and the results were expressed as an index of diabetic glomerulosclerosis (DGS). The glomerular pathology was examined by assessing more than 10 glomerular cross sections per kidney, and the degree of sclerosis in each glomerulus was subjectively graded on a scale of 0–4 where Grade 0 indicates no change; Grade 1 indicates a sclerotic area less than or equal to one-fourth of the glomerulus or a distinct adhesion is present between the capillary tuft and the Bowman’s capsule; Grade 2 indicates sclerosis of one-fourth to one-half of the total glomerular area; Grade 3 indicates sclerosis of more than one-half of the glomerulus but not globally; and Grade 4 indicates global sclerosis.Citation18 DGS was calculated using the following formula:
where N is the number of glomeruli in each grade of sclerosis.
The grade of severity of the DGS was defined as mild with a DGS index ≤2.0, moderate with a DGS index ≥2 and ≤3, or advanced with a DGS index ≥2 and ≤4.0. The damage in the tubular basement membrane and interstitium was evaluated using the point-counting method.Citation19 Under high magnification (400×) with an 81-point (100-square) eyepiece micrometer, we analyzed at least 10 consecutive and nonoverlapping cortical fields from each biopsy section. The points overlying damaged regions in the tubular basement membrane and interstitial space were counted and included in the area percent of damage [tubulointerstitial (TI) damage]. The grade of TI damage was defined as follows: 0, no TI damage; 1+, <25% TI damage; 2+, 25% ≤ TI damage < 50%; and 3+, ≥50% TI damage.
Cluster Analysis
SAS Version 6 (SAS Institute Inc., Cary, NC, USA, 1989) was used for the cluster analysis. The first analysis was performed using red blood cell (RBC) counts, hemoglobin (Hb) levels, and hematocrit (Ht) levels. These patients were divided into four groups with regard to renal prognosis and survival.Citation19 For each group, the following parameters were reviewed at the time of the biopsy as possible candidates for factors that are related to the cluster group showing severe anemia: sex, body mass index [BMI; body weight/(body height)Citation2 (kg/m2)], age (years), systolic blood pressure (BP) (mmHg), diastolic BP (mmHg), duration after the diagnosis of DM (months), serum levels of albumin (mg/dL), blood urea nitrogen (BUN) concentrations (mg/dL), SCr concentrations (mg/dL), the estimated glomerular filtration rate [eGFR (mL/min/·1.73 m2)] that was validated in the Japanese population,Citation20 iron (Fe) levels (μg/dL), total cholesterol levels (mg/dL), triglyceride levels (mg/dL), fasting blood sugar levels (mg/dL), HbA1c (%) levels, urinary protein secretion (g/day), KW nodules, DGS index, and TI grade of damage (0 to +3).
Statistics
The following statistical analyses were performed: χ2-test, Student’s t-test, Kaplan–Meier method, and the generalized Wilcoxon test. The multivariant analyses included a multiple logistic model. p-Values of <0.05 were considered as statistically significant.
RESULTS
In patients with DMN who had SCr concentrations ≤2 mg/dL, a relationship between Ht levels and SCr concentrations was not observed (r = −0.201, p = 0.0246; ). Cluster analysis was performed using RBC counts, Hb levels, and Ht levels, and the cluster groups that differed from each other were then regrouped with regard to renal prognosis (p < 0.0001; ) and survival (p < 0.0187; ). The following factors were significantly associated with the cluster group showing severe anemia in the simple analysis: sex (p = 0.0162), hypoalbuminemia (p < 0.0001), high BUN concentrations (p = 0.0020), eGFR (p = 0.0104; ), and KW nodules (p = 0.0022) in histopathological findings (). In the multifactorial analysis, hypoalbuminemia (p = 0.0277), high BUN (p = 0.0338) concentrations, and low eGFRs (p = 0.0417) were significantly associated with the cluster group showing severe anemia ().
Figure 1. In patients with diabetes mellitus nephrosclerosis (DMN) having serum creatinine (SCr) levels of ≤2 mg/dL, no relationship between hematocrit (Ht) and SCr levels was observed.
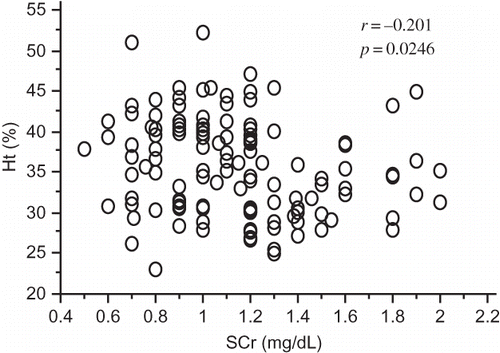
Figure 2. Cluster groups sorted according to the grade of anemia differed significantly with regard to renal prognosis.
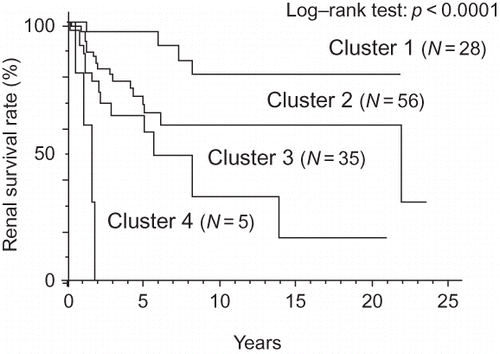
Figure 3. Cluster groups sorted by the grade of anemia differed significantly with regard to survival.
Table 1. Relationship between clinical factors and cluster groups.
Table 2. Relationship between pathological factors and cluster groups.
investigators have not shown improved renal or patient survival with ESA therapy. Therefore, we need to confirm whether early-stage DMN presents with severe anemia without definite renal damage and the early removal of risk factors that cause anemia to progress result in better renoprotectionCitation27 and a decrease in medical expenses.Citation28 Because no relationship was observed between anemia and the severity of renal dysfunction () and because an independence of low GFR and erythropoietin (EPO) secretionCitation21 has been reported, the factors that are related to anemia other than renal dysfunction were analyzed in order to determine whether they influenced anemia in patients with DMN who had SCr concentrations ≤2 mg/dL.
Table 3. Multivariant analysis.
DISCUSSION
The grade of anemia is increased with renal dysfunction,Citation21 and anemia is a risk factor for renal prognosis and survival.Citation2–4,22 In patients with chronic kidney disease (CKD), renal failure, heart failure, and anemia aggravate each other and lead to the development of cardio-renal anemia syndrome.Citation23 In DM patients with CKD stages 1–3, progressive cardiovascular events and renal function progression, as determined by the target Hb levels of 13.0–15.0 g/dL, have not been shown.Citation24 However, the Correction of Hemoglobin and Outcomes in Renal InsufficiencyCitation25 and Trial to Reduce Cardiovascular Events with Aranesp TherapyCitation26
A variety of mechanisms have been suggested for the development of anemia in patients with early-stage DMN. These include EPO secretory cell reduction by TI damage,Citation3 low functionalCitation12 EPO secretion due to autonomic nervous system damage from diabetes,Citation14,15 and EPO loss into the urine due to nephritic syndrome.Citation14,15 Katavetin et al.Citation13 reported that high glucose levels blunt the vascular endothelial growth factor response to hypoxia, or, that is, block EPO production. In this study, the female gender, hypoalbuminemia, high BUN concentrations and low eGFRs in laboratory findings, and KW nodules in histopathological findings were significantly related to anemia in DMN patients with SCr concentrations ≤2 mg/dL by the simple analysis. Furthermore, a multifactorial analysis clarified that hypoalbuminemia, high BUN concentrations, and low eGFRs were significant risk factors for anemia.
Heavier proteinuria will result in hypoalbuminemia in patients in Cluster 4, but heavier proteinuria does not occur in Cluster 3. We therefore suggest that hypoalbuminemia is an important factor other than renal dysfunction that influenced anemia. Generally, a low-protein and high-calorie diet is recommended for patients with renal dysfunction. In DMN patients with CKD stages 4–5, low-protein diet therapy is effective for delaying the start of hemodialysis.Citation29 In contrast, a low-protein diet that is designed to decrease proteinuria does not ameliorate renal dysfunction for patients with DMN and CKD stages 1–3.Citation30 Therefore, an appropriate protein intake volume that corresponds to the CKD stageCitation31 is required, and, furthermore, the results of our study suggest that we must consider the grade of anemia in order to avoid hypoalbuminemia.Citation32 Malnutrition occurs in response to oxidant stress in patients with DMN, and hypocholesteremia and hypoalbuminemia gradually progress. Because the occurrence of malnutrition–inflammation complex syndrome and/or malnutrition–inflammation–atherosclerosis syndromeCitation33 has been reported, malnutrition leads to inflammation, atherosclerosis, and cardiovascular events. The production of inflammatory cytokines, such as interleukin-1, tumor necrosis factor-α, interferon-γ, and interleukin-6, induces the suppression of the multiplication of erythroblast precursor cells, reduces EPO production, and produces anemia. In addition, during inflammation, the secretion of hepcidin as an Fe control hormone accelerates.Citation34 As a result, the supply of Fe is suppressed, and the so-called functional Fe deficiency occurs. Thus, we need to check ferritin and transferrin saturation levels in order to determine the state of the Fe supply in the future.
In this study, there were a number of limitations, including the lack of a control group that was age matched and sex matched, the retrospective nature of the study, the small sample size (especially for patients in Cluster 4), and it was limited to the Japanese population, and, so, it may not be translatable to other populations. Thus, we must investigate this issue in future studies that address these limitations.
In conclusion, we showed in this study that hypoalbuminemia could be associated with renal anemia. Hypoalbuminemia should be recognized as a risk factor for severe anemia, and we should strive to improve conditions of malnutrition and/or alleviate chronic inflammation in these patients. As a result, the progression of anemia may be reduced, and the survival of patients with DMN should be improved.
ACKNOWLEDGMENT
This study was supported in part by a grant for the Progressive Renal Diseases Research Projects from the Ministry of Health, Labor and Welfare, Japan.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- The statistic analyzed commission of Japan Society for Dialysis Therapy: An overview of dialysis treatment in Japan (as of December 31, 2008): 12.
- Sasatomi Y, Kaneoka H, Abe Y, . Anemia and hypertension are risk factors for both renal prognosis and survival in patients with diabetes mellitus. Clin Exp Nephrol. 2009;13:473–479.
- Inomata S, Itoh M, Imai H, Sato T. Serum levels of erythropoietin as a novel marker reflecting the severity of diabetic nephropathy. Nephron 1997;75:426–430.
- Keane WF, Brenner BM, de Zeeuw D, . The risk of developing end stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL Study. Kidney Int. 2003;63:1499–1507.
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75.
- Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Remission and regression in the nephropathy of type 1 diabetes when blood pressure is controlled aggressively. Kidney Int. 2001;60:277–283.
- Hansen HP, Tauber-Lassen E, Jensen BR, Parving HH. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002;62:202–228.
- Remuzzi G, Schieppati A, Ruggenenti P. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346:1145–1151.
- Shestakova MV, Chugunova LA, Shamkhalova MSh, Dirochka IuA, Dedov II. Diabetic nephropathy: Risk factors of rapid progression of renal failure. Ter Arkh. 1999;71:45–49.
- Ravanan R, Spiro JR, Mathieson PW, . Impact of diabetes on hemoglobin levels in renal disease. Diabetologia 2007;50:26–31.
- Li Vecchi M, Fuiano G, Francesco M, . Prevalence and severity of anaemia in patients with type 2 diabetic nephropathy and different degrees of chronic renal insufficiency. Nephron Clin Pract. 2007;105:c62–c67.
- Thomas MC, Tsalamandris C, Macisaac R, Jerums G. Functional erythropoietin deficiency in patients with type 2 diabetes and anaemia. Diabet Med. 2006;23:502–509.
- Katavetin P, Miyata T, Inagi R, . High glucose blunts VEGF response to hypoxia via the oxidative stress-regulated HIF-HRE pathway. J Am Soc Nephrol. 2006;17:1405–1413.
- Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diab Care. 2001;24:495–499.
- Bayes B, Serra A, Junca J, Lauzurica R. Successful treatment of anemia of nephrotic syndrome with recombinant human erythropoietin. Nephrol Dial Transplant. 1998;13:1894–1895.
- Lorber D, Reddan D. Clinical characteristics of chronic kidney disease patients with and without diabetes: A subanalysis of the PAERI study. Clin Nephrol. 2006;66:11–16.
- Ishimura E, Nishizawa Y, Okuno S, . Diabetes mellitus increases the severity of anemia in non-dialyzed patients with renal failure. J Nephrol. 1998;11:83–86.
- Furuta T, Saito T, Ootaka T, . The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21:480–485.
- Sasatomi Y, Sato H, Chiba Y, . Prognostic factors for renal amyloidosis: A clinicopathological study using cluster analysis. Int Med. 2007;46:213–219.
- Hishida A, Yokoyama H, Tomino Y, . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. Epub 2009;53:982–992.
- Kazmi WH, Kausz AT, Khan S, . Anemia: An early complication of chronic renal insufficiency. Am J Kidney Dis. 2001;38:803–812.
- Vlagopoulos PT, Tighiouart H, Weiner DE, . Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: The impact of chronic kidney disease. J Am Soc Nephrol. 2005;16:3403–3410.
- Silverberg DS, Wexler D, Blum M, Wollman Y, Iaina A. The cardio-renal anemia syndrome: Does it exist? Nephrol Dial Transplant. 2003;18(Suppl. 8):S7–S12.
- Ritz E, Laville M, Bilous RW, . Anemia correction in diabetes study investigators. Target level for hemoglobin correction in patients with diabetes and CKD: Primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis. 2007;49:194–207.
- Singh AK, Szczech L, Tang KL, . Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098.
- Pfeffer MA, Burdmann EA, Chen CY, . A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032.
- Kuriyama S, Tomonari H, Yoshida H, Hashimoto T, Kawaguchi Y, Sakai O. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron 1997;77:176–185.
- Lee H, Manns B, Taub K, . Cost analysis of ongoing care of patients with end stage renal disease: The impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40:611–622.
- Nakao T, Kanazawa Y, Matsumoto H, Okada T, Nagaoka Y. Effective dietary protein restriction in the treatment of diabetic nephropathy. Nephrol Front. 2005;4:166–167.
- Pan Y, Guo LL, Jin HM. Low-protein diet for diabetic nephropathy: A meta-analysis of randomized controlled trial. Am J Clin Nut. 2008;88:660–666.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diab Care. 2009;32(Suppl. 1):s13–s61.
- Nakao T, Okada T. Meaning and problems in low-protein diet for renal failure. Jpn J Soc Int Med. 2006;95:368–373.
- Stenvinkel P, Heimbürger O, Wang T, Elinder C-G, Bergström J, Lindholm B. A syndrome of malnutrition, inflammation and atherosclerosis(MIA) is associated with elevated serum hyaluronan and increased mortality in chronic renal failure (CRF). J Am Soc Nephrol. 1999;10:182A.
- Kato A. Increased hepcidin-25 and erythropoietin responsiveness in patients with cardio-renal anemia syndrome. Future Cardiol. 2010;6:769–771.