Abstract
Background and Aims: Acute hepatic injury causes systematic inflammatory responses which may finally lead to functional disturbances in remote organs. In this study, the effects of an inhibitor of inflammatory cytokines (pentoxifylline, PTX) and a well-known antioxidant, N-acetylcysteine (NAC), were evaluated on renal damage and oxidative stress following liver ischemia reperfusion (IR). Method: Five groups of six male rats were used. Group 1 was sham operated. In group 2, 90 min liver partial ischemia was induced by a clamp around both hepatic artery and portal vein and then followed by 4 h of reperfusion. In groups 3 and 4, PTX or NAC was injected intraperitoneally before the ischemia, while in group 5 both drugs were co-administered. The levels of alanine amino-transferase (ALT), aspartate amino-transferase (AST), blood urea nitrogen (BUN), and creatinine in serum as well as malonyldialdehyde (MDA) and glutathione (GSH) levels and morphological changes in renal tissues were assessed. Results: Significant increase in the serum levels of ALT and AST in IR group is indicative of liver functional damages. Elevated BUN and renal tissue MDA, decreased GSH levels, and morphological damages in IR group demonstrate a significant kidney injury and oxidative stress comparing to sham group. Administration of PTX alone and PTX + NAC prevented the IR-induced increase in renal MDA levels. Administration of both drugs and their co-administration prevented the reduction in renal GSH levels and morphological changes. Conclusion: Pretreatment with PTX and NAC before liver IR may be useful to ameliorate renal oxidative damage by preservation of cellular GSH concentration and a reduction in MDA levels.
INTRODUCTION
Hepatic ischemia reperfusion (IR) injury occurs in many occasions including liver transplantation, liver trauma, and hepatectomy.Citation1 Hepatic IR is shown to frequently result in remote organ injury including damages to the kidneys, lung, and heart. In particular, acute kidney injury (AKI) after liver IR is common. The development of AKI after liver injury greatly increases mortality and morbidity in patients.Citation2 Injury to remote organs has been attributed to oxidative stress mediators and other remotely released factors, including proinflammatory cytokines, tumor necrosis factor (TNF), and interleukin-1. However, the mechanisms underlying this response are poorly understood.Citation3
IR injury of the liver has two distinct phases that contribute to hepatocyte damage. The acute phase is characterized by Kupffer cell production of reactive oxygen species (ROS) which results in moderate hepatocyte injury. The later phase includes a cascade of inflammatory events resulting in neutrophil infiltration of the post-ischemic liver. Accumulated neutrophils cause substantial injury to hepatocytes through their release of oxidants and proteases.Citation4 In addition, acute hepatic damage is caused by hepatic IR, which is known to lead to a systemic inflammatory response that results in impaired function of remote organs. Hepatic ischemia has been documented to cause inflammatory lung injury.Citation5 In other studies, acute myocardial injury Citation6 and renal injury was attributed to hepatic ischemia.Citation7 Pentoxifylline (PTX), a non-specific phosphodiesterase inhibitor, has been shown to improve tissue oxygenation and endothelial function as well as inhibiting proinflammatory cytokine production.Citation8 PTX also inhibits cell proliferation and extracellular matrix accumulation. Human studies have proved its antiproteinuric effect in patients with glomerular diseases.Citation9 N-Acetylcysteine (NAC) is an antioxidant that acts by increasing intracellular glutathione (GSH) levels and also by the direct scavenging of ROS such as hypochlorous acid, hydrogen peroxide, superoxide, and hydroxyl radical.Citation10 Thus, in the present study we evaluated the possible involvement of oxidative stress in the liver IR-induced renal injury in rats by examining the effects of PTX, NAC, and their combination.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (250–300 g) were housed in standard conditions (12 h light/day cycle with 20–22°C temperature and 40–50% humidity) and had free access to commercial chow and water. Animal care was in compliance with the guidelines of the Animal and Human Ethical Committee of Tehran Medical Sciences University.
Surgical Protocol
Five groups of rats (n = 6) were included in this study and randomly assigned into one of the experimental groups: sham-operated group (group 1); 90 min liver ischemia and 4 h reperfusion (group 2); in groups 3 and 4, PTX (40 mg/kg) or NAC (400 mg/kg) was injected intraperitoneally before the ischemia, while in group 5 both drugs were co-administered. The levels of alanine amino-transferase (ALT), aspartate amino-transferase (AST), blood urea nitrogen (BUN), and creatinine (Cr) in serum as well as malonyldialdehyde (MDA) and GSH levels in renal tissues were measured. Morphological changes in the kidneys were evaluated by light microscopy.
Rats were placed on a warming pad and were anesthetized by a combination of ketamine (75 mg/kg; Rotex Medica, Trittau, Germany) and xylazine (10 mg/kg; Alfasan, Woerden, The Netherlands). A tracheotomy was performed to facilitate free breathing. After tracheotomy, tail vein was cannulated (using Venflon 22GA, 0.98IN, ID 0.8 mm, Helsingborg, Sweden) and 0.9% normal saline was infused to maintain euvolemia. Systolic blood pressure was measured by the tail-cuff method connected to a pneumatic transducer using a PowerLab/4sp data acquisition system (software Chart, version 5, AD Instruments, Castle Hill, Australia). Body temperature was maintained at 37 ± 1°C. A midline laparotomy was performed and liver artery and portal vein was carefully separated from the surrounding tissues. To induce partial ischemia, portal vein and liver artery perfusing left and median lobes of the liver were occluded by a non-traumatic microvascular clip (Beimer-Clip, Aesculap, Germany).
Sham-operated animals underwent identical surgical treatment, including isolation of the liver artery. However, artery occlusion was not performed. After completion of the surgery, rats were allowed to stabilize for 30 min.
Ninety minutes of liver ischemia was induced and rats were killed after 4 h of reperfusion. Blood samples were collected and centrifuged at 4000 × g for 10 min at 4°C, and serum was collected for chemical analysis. Kidney tissues were fixed in formalin (10% phosphate-buffered, pH = 7.4) for histological evaluations. Remainders of renal tissues were washed in cold phosphate-buffered saline and snap-frozen in liquid nitrogen. The samples were stored at −70°C until further studies.
Biochemical Assay
BUN and Cr were used as renal functional indices. Blood concentrations of AST and ALT were determined by commercially available kits to confirm IR-induced injury to the liver.
Measurement of Renal Oxidative Stress Markers
The tissue MDA level was determined by the method of Esterbauer and Cheeseman based on its reaction with thiobarbituric acid (TBA) at 90–100°C and the measurement of the absorbance at 532 nm.Citation11 MDA reacts with TBA and produces a pink pigment which has a maximum absorption at 532 nm. The value of each sample was obtained from the standard curve and was expressed as nmol/g tissue. Renal GSH concentration was assessed as a measure of nonenzymatic antioxidant. Total GSH concentration of kidney tissues was measured according to the modified method of Tietze by Griffith which was based on the conversion of 5,5′-dithiobis-2-nitrobenzoic acid to 5-thio-2-nitro benzoate by nicotinamide adenine dinucleotide phosphate in the presence of GSH reductase. Formation of 5-thio-2-nitrobenzoate was measured by spectrophotometry at 412 nm.Citation12,13
Histological Analysis
After formalin fixation (10% phosphate-buffered) and dehydration, paraffin-embedded renal sections (4 μm) were stained by hematoxylin and eosin. Tubules were evaluated for the presence of degenerative changes (vacuolization), tubular dilatation, luminal debris, cast formation, and loss of brush borders from proximal tubules.
Statistical Analyses
Data are expressed as the mean ± SEM. Comparisons between groups were made by one-way analysis of variance followed by the post hoc Tukey test. The SPSS software (Statistical Package for the Social Sciences, version 13.0, SPSS Inc., Chicago, IL, USA) was used for data analysis. p-Values <0.05 were considered significant.
RESULTS
Liver Functional Changes
Liver ischemia for 90 min followed by 4 h reperfusion resulted in significant increase in liver injury demonstrated by an increase in serum concentrations of ALT (9630 ± 196 vs. 309 ± 36.3 U/L, p < 0.05) and AST (6740 ± 690 vs. 410 ± 43.4 U/L, p < 0.05) compared to sham-operated rats.
Renal Functional Changes
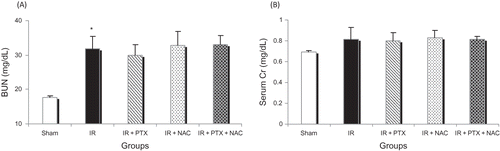
Liver ischemia for 90 min followed by 4 h reperfusion resulted in a significant rise in BUN but no change in serum Cr concentrations compared to sham-operated rats (). There were no significant changes in serum Cr and BUN in PTX or NAC alone and PTX + NAC combination groups compared to IR group (). There were also no significant differences in serum Cr and BUN between PTX and NAC alone and PTX + NAC combination groups ().
Renal Oxidative Status Changes
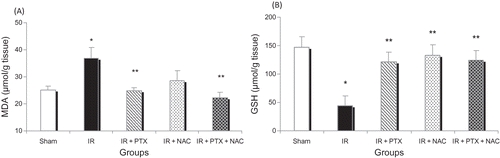
Liver ischemia for 90 min followed by 4 h reperfusion resulted in significant changes in renal oxidative stress markers including increase in renal MDA (A) and decrease in renal GSH levels (B) compared to sham-operated rats. Administration of PTX alone and PTX + NAC prevented the IR-induced increase in renal MDA levels (A). Administration of both drugs and their co-administration prevented the reduction in renal GSH levels (B). There were no significant differences in the above indices between PTX and NAC alone and PTX + NAC combination groups ().
Histological Evaluations
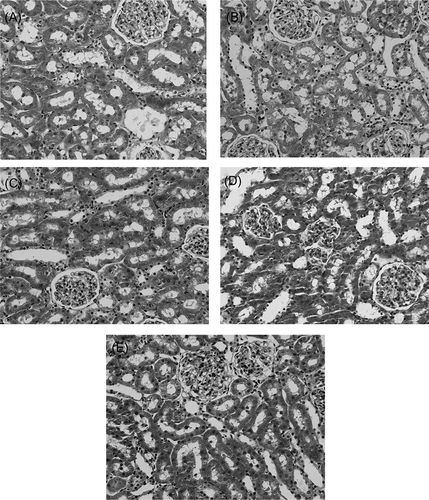
As shown in , there are no detectable changes using light microscopy in the kidney of sham group (A). Cells are healthy, nuclei are normal, the brush borders are intact, and tubular lumens are open. In liver ischemia for 90 min followed by 4 h reperfusion (B), there is significant alteration in the kidney histology, including formation of luminal debris, flattening of tubular cells, cellular vacuolization, irregularities in cytoplasm and nuclei of epithelial cells, and loss of brush borders. In NAC group, there was partial recovery in tubular cells compared to IR group (D). Similarly, in PTX alone and PTX + NAC combination groups (C and E), there were less structural damage compared to IR group. This was demonstrated by less cytoplasmic vacuolation and lower tubular debris in a way that most of the tubules are open.
DISCUSSION
The present study demonstrates that hepatic ischemia for 90 min followed by 4 h reperfusion results in partial impairment of renal function as well as morphological changes and oxidative stress of the renal tissues. Pretreatment with PTX, NAC, and their combination significantly reduced renal oxidative stress.
Hepatic IR is known to induce remote organ dysfunction in the heart and lung.
The incidence of renal dysfunction following liver transplantation is high and secondary renal dysfunction occurs commonly in chronic hepatic failure.Citation2 Acute hepatic damage due to hepatic IR is also known to cause a systemic inflammatory response that results in the damage of remote organ systems.Citation14
The results of this study as well as similar findings of Behrends et al.Citation15 demonstrate moderate changes in BUN concentrations but no change in serum Cr concentrations in this model of hepatic IR. We can only speculate that the kidney has a higher tolerance for systemic cytokines, resulting in only minimal effects of remote organ ischemia on the kidneys. Cytokines such as TNF-α are known to be the mediators of remote myocardial dysfunction Citation16 and remote lung injury.Citation17 In support of this hypothesis, the cytokine concentrations seen in septic shock, which are considerably higher than those seen after hepatic IR, rarely result in acute tubular necrosis.Citation18 An explanation for this resistance against remote renal injury was presented in a study by Tanaka et al. which demonstrated that hepatic IR resulted in upregulation of renal heme oxygenase-1 (HO-1).Citation1 The known cytoprotective effects of HO-1 might be responsible for the prevention of massive reduction in renal function.
ROS are critical mediators of the early phase of ischemic (IR) injury. Overproduction of these free radicals induces an increase in lipid peroxidation (MDA) by destroying unsaturated fatty acids in the cell membrane, causing a decrease in endogenous antioxidants such as GSH in renal tissues.Citation19 In this study, administration of PTX and NAC to rats resulted in the preservation of GSH concentration. PTX administration also caused a significant reduction in MDA levels compared to IR group. Nitescu et al. showed that NAC improves kidney function and reduces renal interstitial inflammation in rats subjected to renal IR injury. These effects were associated with increased renal GSH levels and decreased plasma ascorbyl concentrations, suggesting that NAC is able to attenuate renal as well as systemic oxidative stress in this model.Citation10
In the present study, GSH levels decreased during IR while administration of NAC preserved GSH levels. The contribution of antioxidants, such as NAC, in ameliorating the parenchymal lesions, inflammatory parameters, and functional variables in renal IR is still controversial. The protective effect of NAC was better observed at high concentrations and early period of times.Citation20 In addition, beneficial effects of combination of NAC and a scavenger of peroxynitrite on renal ischemia/reperfusion injury were observed in a recent study by Kizilgun et al. in 2011.Citation21
IR injury is a biphasic phenomenon. The first phase is ischemia that initiates the tissue injury by causing energy deprivation, which may ultimately lead to cellular dysfunction and death. Reperfusion, as the second phase, is more important but may exacerbate this damage by inflammatory reactions, production of oxygen free radicals, and release of endothelial factors.Citation22 Previous studies have shown that NAC, PTX, and/or multi-drug administration prevented hepatic reperfusion injury.Citation23,24
PTX is a hemorheologic agent used for patients with vascular disease and other conditions caused by defects in regional microcirculation.Citation25 PTX prevents increases of microvascular permeability and formation of edema.Citation26 In addition, PTX prevents ischemic vasoconstriction.Citation27,28 NAC also promotes vasodilatation and improves blood flow in microcirculation.Citation29,30
The lower morphological damage in PTX-treated kidneys suggests a reduction in oxidative stress in this group. PTX, a xanthine derivative, which is a well-known suppressor of TNF-α production from inflammatory cells, has also been shown to inhibit the growth of hepatic stellate cells and inhibit collagen synthesis in these cells.Citation31 Recent reports suggest that PTX can enhance the chemotactic response of neutrophils and may inhibit phagocytosis and superoxide production by neutrophils and monocytes.Citation32 PTX has been shown to improve tissue oxygenation and endothelial function and to inhibit proinflammatory cytokine production.Citation8 In the present study, PTX improved tissue oxygenation by a reduction in oxidative stress but this was not sufficient for preservation of renal function. Although downregulation of proinflammatory cytokines seems to be important for the protection or attenuation of injury it is possible that mitochondria were already highly damaged by the ischemic process. Previous studies have shown a beneficial effect of 50 mg/kg PTX supplementation to correct the overproduction of oxidative stress and inflammatory indices in IR-induced spinal cord injury and fatty liver diseaseCitation33,34 and a preventive effect for 400 mg/kg NAC was demonstrated in the rat organs against the oxidative damages.Citation35,36
Results of this study also demonstrated that beneficial effects of PTX were more than NAC. This suggests the importance of inflammatory factors among other mediators in the induction of renal damage. Previous studies have demonstrated that glomerular filtration rate was significantly improved by PTX pretreatment in renal IR and this protective effect of PTX on functional changes was supported by morphological studies. The expression of TNF-α mRNA was increased after reperfusion, which was inhibited by PTX pretreatment. These results suggest that PTX may exert a protective effect against ischemic acute renal failure by inhibiting the production of TNF-α in rabbits.Citation37
In conclusion, the significant increase in MDA levels, decrease in GSH, and destructive appearance in histology accompanied by partial impaired function in the kidney suggested that hepatic IR-induced renal injury may be mediated through oxidative reactions. In addition, PTX and NAC administrations protected kidney tissue against this oxidative damage caused by hepatic IR injury.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Tanaka Y, Maher JM, Chen C, Klaassen CD. Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Mol Pharmacol. 2007;71(3):817–825.
- Davis CL, Gonwa TA, Wilkinson AH. Pathophysiology of renal disease associated with liver disorders: Implications for liver transplantation. Part I. Liver Transpl. 2002;8(2):91–109.
- Miranda LE, Capellini VK, Reis GS, Celotto AC, Carlotti Jr. CG, Evora PR. Effects of partial liver ischemia followed by global liver reperfusion on the remote tissue expression of nitric oxide synthase: Lungs and kidneys. Transplant Proc. 2010;42(5):1557–1562.
- Okaya T, Lentsch AB. Cytokine cascades and the hepatic inflammatory response to ischemia and reperfusion. J Invest Surg. 2003;16(3):141–147.
- Weinbroum AA, Hochhauser E, Rudick V, . Direct induction of acute lung and myocardial dysfunction by liver ischemia and reperfusion. J Trauma. 1997;43(4):627–633; discussion 33–35.
- Hochhauser E, Ben-Ari Z, Pappo O, Chepurko Y, Vidne BA. TPEN attenuates hepatic apoptotic ischemia/ reperfusion injury and remote early cardiac dysfunction. Apoptosis. 2005;10(1):53–62.
- Park SW, Kim M, Chen SW, D’Agati VD, Lee HT. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock. 2010;33(1):31–42.
- Okumura AS, Rodrigues LE, Martinelli R. Pentoxifylline in ischemia-induced acute kidney injury in rats. Ren Fail. 2009;31(9):829–832.
- Lin SL, Chen YM, Chiang WC, Wu KD, Tsai TJ. Effect of pentoxifylline in addition to losartan on proteinuria and GFR in CKD: A 12-month randomized trial. Am J Kidney Dis. 2008;52(3):464–474.
- Nitescu N, Ricksten SE, Marcussen N, . N-acetylcysteine attenuates kidney injury in rats subjected to renal ischemia-reperfusion. Nephrol Dial Transplant. 2006;21(5):1240–1247.
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421.
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–212.
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–522.
- Colletti LM, Burtch GD, Remick DG, . The production of tumor necrosis factor alpha and the development of a pulmonary capillary injury following hepatic ischemia/reperfusion. Transplantation. 1990;49(2):268–272.
- Behrends M, Hirose R, Park YH, . Remote renal injury following partial hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg. 2008;12(3):490–495.
- Lu X, Hamilton JA, Shen J, . Role of tumor necrosis factor-alpha in myocardial dysfunction and apoptosis during hindlimb ischemia and reperfusion. Crit Care Med. 2006;34(2):484–491.
- Souza DG, Cassali GD, Poole S, Teixeira MM. Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischemia and reperfusion injury. Br J Pharmacol. 2001;134(5):985–994.
- Wan L, Bellomo R, Di Giantomasso D, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care. 2003;9(6):496–502.
- Kaynar K, Gul S, Ersoz S, Ozdemir F, Ulusoy H, Ulusoy S. Amikacin-induced nephropathy: Is there any protective way? Ren Fail. 2007;29(1):23–27.
- Di Giorno C, Pinheiro HS, Heinke T, . Beneficial effect of N-acetyl-cysteine on renal injury triggered by ischemia and reperfusion. Transplant Proc. 2006;38(9):2774–2776.
- Kizilgun M, Poyrazoglu Y, Oztas Y, . Beneficial effects of N-acetylcysteine and ebselen on renal ischemia/reperfusion injury. Ren Fail. 2011;33(5):512–517.
- Hamidian Jahromi A, Kessaris N, Sharifian M, Roozbeh J. Protective effect of pentoxifylline in the kidney perfusion fluid on the transplanted kidney. Saudi J Kidney Dis Transpl. 2009;20(2):290–291.
- Moussavian MR, Scheuer C, Schmidt M, . Multidrug donor preconditioning prevents cold liver preservation and reperfusion injury. Langenbecks Arch Surg. 2011;396(2):231–241.
- Nagasaki H, Nakano H, Boudjema K, . Efficacy of preconditioning with N-acetylcysteine against reperfusion injury after prolonged cold ischemia in rats liver in which glutathione had been reduced by buthionine sulphoximine. Eur J Surg. 1998;164(2):139–146.
- Cerqueira NF, Hussni CA, Yoshida WB, Sequeira JL, Padovani CR. Effects of pentoxifylline and N-acetylcysteine on injuries caused by ischemia and reperfusion of splanchnic organs in rats. Int Angiol. 2008;27(6):512–521.
- Coe DA, Freischlag JA, Johnson D, . Pentoxifylline prevents endothelial damage due to ischemia and reperfusion injury. J Surg Res. 1997;67(1):21–25.
- Steeb GD, Wilson MA, Garrison RN. Pentoxifylline preserves small-intestine microvascular blood flow during bacteremia. Surgery. 1992;112(4):756–763; discussion 63–64.
- Tireli GA, Salman T, Ozbey H, Abbasoglu L, Toker G, Celik A. The effect of pentoxifylline on intestinal anastomotic healing after ischemia. Pediatr Surg Int. 2003;19(1–2):88–90.
- Koksal C, Bozkurt AK, Cangel U, . Attenuation of ischemia/reperfusion injury by N-acetylcysteine in a rat hind limb model. J Surg Res. 2003;111(2):236–239.
- Portella AO, Montero EF, Poli de Figueiredo LF, Bueno AS, Thurow AA, Rodrigues FG. Effects of N-acetylcysteine in hepatic ischemia-reperfusion injury during hemorrhagic shock. Transplant proc. 2004;36(4):846–848.
- Toda K, Kumagai N, Kaneko F, . Pentoxifylline prevents pig serum-induced rat liver fibrosis by inhibiting interleukin-6 production. J Gastroenterol Hepatol. 2009;24(5):860–865.
- Oksuz H, Bulbuloglu E, Senoglu N, . Re-protective effects of pre- and post-laparoscopy conditioning, zinc, pentoxifylline, and N-acetylcysteine in an animal model of laparoscopy-induced ischemia/reperfusion injury of the kidney. Ren Fail. 2009;31(4):297–302.
- Savas S, Delibas N, Savas C, Sutcu R, Cindas A. Pentoxifylline reduces biochemical markers of ischemia-reperfusion induced spinal cord injury in rabbits. Spinal Cord. 2002;40(5):224–229.
- Zaitone S, Hassan N, El-Orabi N, El-Awady el S. Pentoxifylline and melatonin in combination with pioglitazone ameliorate experimental non-alcoholic fatty liver disease. Eur J Pharmacol. 2011;662(1–3):70–77.
- Da Silveira M, Yoshida WB. Trimetazidine and N-acetylcysteine in attenuating hind-limb ischemia and reperfusion injuries: Experimental study in rats. Int Angiol. 2009;28(5): 412–417.
- Shimizu MH, Danilovic A, Andrade L, . N-acetylcysteine protects against renal injury following bilateral ureteral obstruction. Nephrol Dial Transplant. 2008;23(10):3067–3073.
- Kim YK, Yoo JH, Woo JS, Jung JS, Kim BS, Kim SY. Effect of pentoxifylline on ischemic acute renal failure in rabbits. Ren Fail. 2001;23(6):757–772.