Abstract
We describe a 32-year-old female with past medical history of preeclampsia, who presented at 29th week of gestation of her second pregnancy with abdominal pain, emesis, and diarrhea. Initial evaluation revealed hypertension, placental abruption, and intrauterine fetal death. After spontaneous rupture of membranes, a stillborn fetus was delivered. The clinical course was complicated by seizures and acute kidney injury requiring hemodialysis. She also exhibited microangiopathic hemolytic anemia, thrombocytopenia, and elevated liver enzymes (consistent with HELLP syndrome). A biopsy showed acute renal cortical necrosis.
INTRODUCTION
HELLP syndrome, characterized by hemolysis, elevated liver enzymes, and low platelet count, is a serious complication of pregnancy, often accompanied by acute kidney injury (AKI). Acute renal cortical necrosis (ARCN) is important to consider when acute renal failure develops in HELLP syndrome. Suspicion is raised when renal function fails to recover. Although the incidence of ARCN has declined substantially, it still remains an important cause of pregnancy-related AKI.
CASE REPORT
A 32-year-old gravida 2 para 1 Caucasian female was admitted at 29th week of gestation with severe abdominal pain, emesis, and non-bloody diarrhea of 8-h duration. She had only mild hypertension during this pregnancy. Her preceding pregnancy was complicated by eclampsia, but she delivered a viable male infant at 29-week gestation. Her mother also had history of pregnancy-induced hypertension. The patient was found to be hypertensive (BP 180/108 mmHg) in the emergency department and was treated with intravenous labetalol and hydralazine. An ultrasound Doppler revealed partial placental abruption and intrauterine death. A stillborn fetus was delivered 40 minutes after spontaneous rupture of membranes. The placenta appeared intact, and no retained products of conception were identified on postpartum ultrasound. The clinical course was further complicated by a generalized tonic-clonic seizure and anuria. Laboratory evaluation within the first day of admission revealed anemia (hemoglobin dropped from 14.8 to 6.9 g/dL), schistocytes on peripheral blood film, and thrombocytopenia (platelet count declined from 183,000 to 62,000). Over the next 4 days, her serum creatinine rose from 1.9 to 6.7 mg/dL (baseline prior to pregnancy 0.8 mg/dL). Persistent anuria and hyperkalemia (serum potassium 6.4 mEq/L) prompted initiation of hemodialysis on day 5. The patient had elevated lactate dehydrogenase (5641 IU/L) and worsening liver functions (serum aspartate transaminase rose from 71 to 692 IU/L, and alanine transaminase from 36 to 292 IU/L). A clinical diagnosis of HELLP syndrome with AKI was made. Patient was treated with supportive measures; blood pressure was well controlled; and hemodialysis was continued. The HELLP parameters normalized over the next few days.
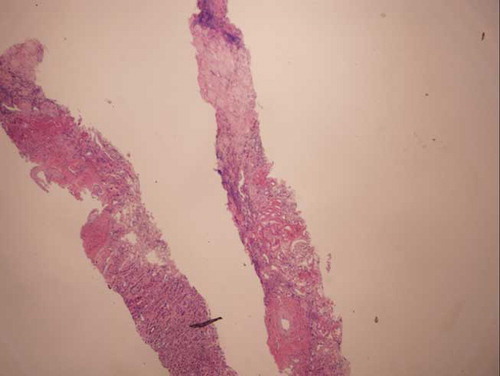
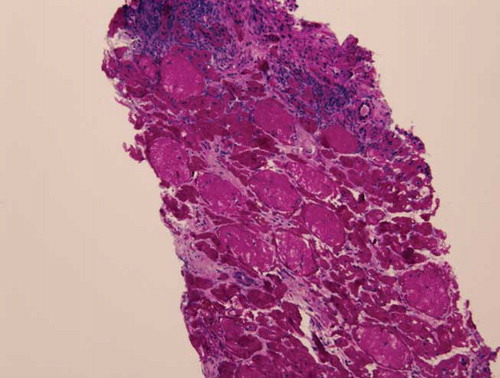
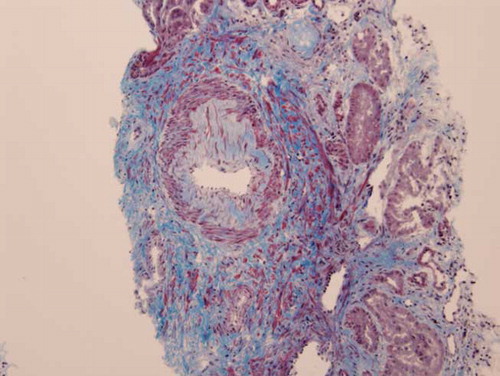
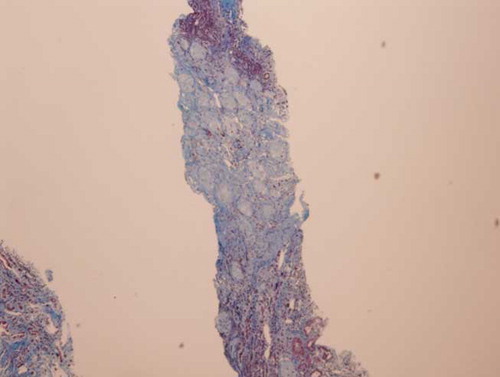
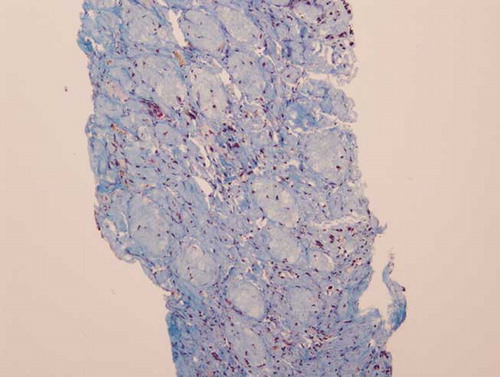
The patient became non-oliguric, and a slight improvement was noted in her renal function after several weeks. She, however, remained dialysis dependent, which prompted a renal biopsy. Renal histopathology revealed closely packed atrophic glomeruli in a back-to-back configuration, suggesting substantial cortical atrophy (). Moderate tubular atrophy, moderate interstitial fibrosis (, , , and ), and intimal fibroplasia involving larger blood vessel were noted (). There was no evidence of microthrombi, arterial hyaline deposition, or fibrinoid necrosis. Immunofluorescence was negative. These findings confirmed the feared diagnosis of ARCN.
DISCUSSION
The HELLP syndrome is a serious complication of pregnancy, characterized by hemolysis, elevated liver enzymes, and low platelet count. It develops in 0.5–0.9% of all pregnancies and in 10–20% of cases with severe preeclampsia.Citation1,2 Thought to be a rare complication only of severe preeclampsia, 20% cases occur in the absence of hypertension and proteinuria.Citation3 HELLP syndrome was found to be associated with AKI 36–50% of the time.Citation4,5 Our patient developed preeclampsia, complicated by HELLP syndrome and AKI.
The commonest renal histology reported in patients with HELLP syndrome and AKI is acute tubular necrosis (ATN).Citation3,6,7 Patients with that pathology alone usually exhibit complete recovery of their renal function.Citation3 Thrombotic microangiopathy, glomerular endotheliosis, nephrosclerosis, and glomerulonephritis also have been noted in patients with HELLP syndrome.Citation6,8–10 These lesions may be present alone or in combination with ATN. ARCN is an uncommon but feared complication of HELLP syndrome.Citation3,11 This entity should be suspected when a patient fails to show recovery in renal function after 4–6 weeks. Our patient eventually became non-oliguric and exhibited a slight improvement in her renal function; however, she remained dialysis dependent. A renal biopsy undertaken to evaluate the potential for recovery revealed advanced renal cortical necrosis.
Clinically, ARCN often results in irreversible renal failure, but the incomplete or patchy variety can present with initial oliguria and even anuria, lasting longer than uncomplicated tubular necrosis, followed by a variable return of renal function and a stable period of moderate renal insufficiency. Some patients unfortunately progress years later to end-stage disease.Citation12 ARCN is most often associated with complications of pregnancy, including septic abortion, abruptio placentae, placenta previa, amniotic fluid embolism, and prolonged intrauterine fetal death.Citation13,14 Non-obstetric conditions including snakebites, hemolytic uremic syndrome, renal trauma, fulminant sepsis, and hyperacute renal transplant rejection are much less frequently associated with ARCN.Citation13,15–17
Placental abruption is the most common obstetric predisposition for ARCN and appears to trigger the same combination of endothelial injury, hypercoagulability, and intravascular thrombosis identified in other causes of ARCN.Citation18,19 Septic abortion is also an important factor predisposing to ARCN. The release of bacterial endotoxin may lead to endothelial activation and injury with subsequent vascular thrombosis and renal ischemia. Placental abruption coupled with underlying hypertension might have contributed to the development of ARCN in our patient.
Preeclampsia is a multi-organ disorder characterized by systemic hypertension and renal, hepatic, and cerebral vascular pathology. These clinical manifestations are consistent with widespread endothelial dysfunction, vasoconstriction, and end-organ ischemia. Abnormal placental angiogenesis during pregnancy resulting from high levels of the anti-angiogenic factors, soluble fms-like tyrosine kinase and soluble endoglin, has been implicated in preeclampsia.Citation20
Redman et al. have suggested that preeclampsia is an extreme end of a continuous spectrum of inflammatory responses that are a feature of pregnancy itself.Citation21 This hypothesis is also supported by studies that demonstrate that when a single dose of endotoxin or interleukins is given to pregnant animals, they manifest clinical features of preeclampsia, but not the controls.Citation22,23
The resulting hemodynamic and endothelial changes superimposed on an already existing state of intravascular volume depletion make the kidneys more vulnerable to develop AKI (ATN and rarely acute cortical necrosis).
Despite the differences in pathophysiology between the most common causes of AKI complicating gestation, ATN, and ARCN, clinical distinction between the two entities can be challenging.Citation24 Profound oliguria and rapidly rising blood urea nitrogen and creatinine can characterize both of these conditions. The clinical setting (septic abortion and placental abruption) and accompanying evidence of intravascular coagulation might point to ARCN, but additional studies may be required to make a definitive diagnosis.
Histologically, ATN and ARCN are clearly separate entities. In ATN, renal biopsy findings are subtle, often limited to the tubulointerstitium, and much different from the catastrophic tissue insult seen in ARCN.Citation24 Biopsy findings early in ARCN reveal ischemic necrosis of the renal cortex due to a combination of vascular spasm, microvascular injury, and intravascular coagulation. It is typically extensive and bilateral, although focal and localized forms may occur. The histological pattern of ARCN is often patchy when the biopsy is undertaken soon after the insult.Citation13,25 The biopsy in our case was done later in the course, and more advanced changes of cortical atrophy were noted, which clearly defined her poor renal prognosis.
Diagnosis of ARCN does not always require renal biopsy, which may also be difficult to perform given the underlying coagulopathy and the clinical instability of many patients. The biopsy may also miss the diagnosis if ARCN is patchy. Other noninvasive diagnostic modalities like ultrasound, radionucleotide scintigraphy, computerized tomography (CT) scans, magnetic resonance imaging, and renal arteriography each has specific features that support the clinical diagnosis of ARCN. Contrast-enhanced CT scan provides very characteristic findings of reduced contrast uptake in the cortices, and in later stages cortical calcification may be detected.
In summary, ARCN remains an important differential in the diagnosis of AKI in pregnant patients. Although the devastating disease can present similarly to ATN, there are clear histologic and pathophysiological distinctions. This endothelial-driven injury is characterized by patchy or diffuse cortical necrosis, ultimately resulting in tightly packed glomeruli with severe tubulointerstitial atrophy. The alterations of the endothelium that characterize pregnancy itself provide a fertile environment to develop complications like preeclampsia, HELLP syndrome, and ARCN.
Although often partially reversible, patients not infrequently require long-term renal replacement therapy. With supportive care, however, 20–40% of patients recover enough renal function to become dialysis independent.13
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. Preeclampsia: A renal perspective. Kidney Int. 2005;67:2101–2113.
- Geary M. The HELLP syndrome. Br J Obstet Gynaecol. 1997;104:887–891.
- Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Freidman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169(4): 1000–1006.
- Secluk NY, Odabas AR, Cetinkaya R, Tonbul HZ, San A. Outcome of pregnancies with HELLP syndrome complicated by acute renal failure (1989–1999). Ren Fail. 2000;22(3): 319–327.
- Drakley AJ, Le Roux PA, Anthony J, Penny J. Acute renal failure complicating severe preeclampsia requiring admission to an obstetric intensive care. Am J Obstet Gynecol. 2002;186: 253–256.
- Pourrat O, Touchard G, Robert R, . A kidney biopsy is clearly mandatory to confirm the indication of plasma exchange in adult hemolytic-uremic syndrome. Ann Med Int. 1994;145:369–372.
- Ghosh AK, Vashisht K, Varma S, Khullar D, Sakhuja V. Acute renal failure in a patient with HELLP syndrome—An unusual complication of eclampsia. Ren Fail. 1994;16:295–298.
- Kahra K, Draganov B, Sund S, Hovig T. Post partum renal failure: A complex case with probable co-existence of hemolysis, elevated liver enzymes, low platelet count, and hemolytic uremic syndrome. Obstet Gynecol. 1998;92:698–700.
- Beller FK, Dame WR, Ebert C. Pregnancy induced hypertension complicated by thrombocytopenia, hemolysis and elevated liver enzymes (HELLP) syndrome: Renal biopsies and outcome. Aust N Z J Obstet Gynaecol. 1985;25:83–86.
- Fang J, Chen Y, Huang C. Unusual presentation of mesangial proliferative glomerulonephritis in HELLP syndrome associated with acute renal failure. Ren Fail. 2000;22:641–646.
- Mohaupt MG. Anuria after delivery. Ther Umsch. 1998;55: 579–582.
- Pertuiset C, Grunfeld JP. Acute renal failure in pregnancy. Clin Obstet Gynaecol. 1994;8:333–351.
- Matlin RA, Gary NF. Acute cortical necrosis: Case report and review of literature. Am J Med. 1974;56:110–118.
- Grunfeld JP, Ganeval D, Bowmerias F. Acute renal failure in pregnancy. Kidney Int. 1980;18:179.
- Ali SS, Rizvi ZS, Muzaffar S, Ahmad A, Ali A, Hassan HH. Renal cortical necrosis: A case series of nine patients and review of literature. J Ayub Med Coll Abbottabad. 2003;15:1–4.
- Sakhuja V, Chugh KS. Renal cortical necrosis. Int J Artif Organs. 1986;9:145–146.
- Chugh KS, Singhal PC, Kher VK, . Spectrum of acute cortical necrosis in Indian patients. Am J Med Sci. 1983;283:10–20.
- Lauler DP, Schreiner GE. Bilateral cortical necrosis. Am J Med. 1958;24:519.
- Sibai B, Ramadan M. Acute renal failure in pregnancies complicated by hemolysis, elevated liver enzymes, and low platelets. Am J Obstet Gynecol. 1993;168:1682–1687.
- Bdolah Y, Skhatme VP, Karumanchi SA. Angiogenic imbalance in the pathophysiology of preeclampsia: Newer insights. Semin Nephrol. 2004;24(6):548–556.
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506.
- Faas MM, Schuling GA, Baller JF, Visscher CA, Bakker WW. A new animal model for human preeclampsia: Ultra low dose endotoxin infusion in pregnant rats. Am J Obstet Gynecol. 1994;171:158–164.
- Hayakawa S, Fujikawa T, Fukuoka H, . Murine fetal resorption and experimental preeclampsia are induced by both excessive Th1 and Th2 activation. J Reprod Immunol. 2000;41:121–138.
- Rosen S, Stillman I. Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol. 2008;19:871–875.
- Kleinknecht D, Grunfeld JP, Gomez PC, Moreau JF, Garcia-Torres R. Diagnostic procedure and long term progress in bilateral cortical necrosis. Kidney Int. 1973;4:390.