Abstract
The survival of patients undergoing peritoneal dialysis (PD) has improved over the past decade, but their mortality rate remains high. The aim of the current study was to identify correctable and uncorrectable factors influencing survival according to the elapsed time in patients undergoing PD. We retrospectively analyzed data from medical records of 118 patients who had undergone PD for >6 months. We analyzed laboratory findings at three time points (point of PD initiation, 6-month point of PD and 3-month point prior to death or last follow-up) during PD treatment and prescribed medications taken for >50% of the follow-up period. Three-year survival group was younger, had lower prevalence rates of ischemic heart disease (p = 0.024) and heart failure (HF) (8.5% vs. 34.6%, p = 0.003), higher serum albumin levels (albumin 2) at the 6-month point of PD, and higher serum albumin (albumin3) and creatinine (creatinine3) levels at the 3-month point prior to death or last follow-up than nonsurvival group. Patients without underlying HF, patients treated with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers during the last 6 months (p = 0.0042), and those having creatinine 3 >9.5 mg/dL (p = 0.0029), and albumin 2 (p = 0.0209) and albumin 3 >3.5 g/dL (p = 0.0003) showed significantly higher survival curves. HF and albumin 3 were found to be independent factors for 3-year survival and long-term survival, respectively, by the multivariate Cox regression model. In conclusion, HF was useful information for predicting 3-year survival, and low serum albumin levels according to elapsed time should be corrected for survival of PD patients.
INTRODUCTION
Peritoneal dialysis (PD) is an established renal replacement method for end-stage renal disease and is considered to be more valuable for maintaining residual renal function and reducing cardiovascular instability than hemodialysis (HD). However, PD is not more favorable than HD with respect to survival after the first 2 years of dialysis treatment.Citation1,2 In addition, cardiovascular death after 1 year of dialysis treatment is much increased in PD patients compared with HD patients.Citation3 Mortality risk after dialysis was 8% lower for PD than for propensity-matched HD patients in another study.Citation4 However, this survival benefit of PD was significantly maintained for up to 24 months and found among subgroups without cardiovascular disease (CVD). Therefore, based on these findings, further studies will be needed for improving 2- or 3-year survival in PD patients.
The survival rate for patients undergoing PD has improved recently.Citation5 United States Renal Data System data from 2003 reported a 5-year survival rate of 32.7% in PD patients who initiated treatment from 1994 to 1998, which was a 17% increase over the previous time period (1989–1993).Citation6 It has been reported that after adjustment for age, gender, diabetes mellitus (DM), and cardiovascular comorbidities, patient survival significantly improved during 1992–2005 compared with that during 1981–1992.Citation7 However, the current mortality rate of PD patients remains high, although survival of PD patients has improved over the past decades. Therefore, it is necessary to overcome the low survival rate by evaluating correctable risk factors.
A number of clinical studies have attempted to confirm the factors affecting the survival of patients undergoing PD and reported that age, malnutrition, electrolyte unbalance, comorbid diseases such as diabetes and CVD, and residual renal function were related to PD mortality.Citation8–11 However, there are few reports concerning factors affecting mortality according to elapsed time after PD. In most studies, risk factors influencing mortality were evaluated at the time of PD initiation. The aim of the current study was to identify correctable and uncorrectable factors influencing survival according to the elapsed time in PD patients. In addition, we tried to identify factors related to 3-year survival by analyzing laboratory findings at several time points and the medications prescribed during PD treatment.
PATIENTS AND METHODS
Patients
We retrospectively analyzed data from the medical records of 391 patients who started PD treatment in our hospital between October 1999 and September 2008. We excluded patients who had previously been treated with renal replacement therapy or had been switched to HD or kidney transplantation because we tried to investigate factors associated with PD treatment as a first renal replacement therapy. We excluded patients who had dropped out at our hospital, had a malignancy, or had liver cirrhosis (>Child B). We also excluded patients who had not maintained PD treatment for >6 months, because we tried to exclude factors associated with acute technical failure or acute medical complications by delayed dialysis. We also excluded patients who had not followed up for >3 years among PD patients who were alive, because we tried to identify factors among patients who were alive for >3 years. Finally, 118 patients were analyzed, and they began PD treatment in our center and maintained PD treatment for >3 years or died after PD treatment for at least 6 months.
Methods
At the initiation of PD, the following demographic and comorbid characteristics were collected: age, gender, body mass index (BMI), and underlying diseases including DM, hypertension (HTN), ischemic heart disease (IHD), and heart failure (HF). We defined IHD patients as those with previous diagnosis by coronary angiography, echocardiography, electrocardiographic changes, and elevated troponin-I, or myocardial single-photon emission computed tomography scan. HF was defined as an ejection fraction of <50% on a previous echocardiography. We reviewed systolic and diastolic blood pressures at PD initiation and at the 3-month point prior to death or last follow-up. We analyzed the laboratory findings at three time points during PD treatment (point 1, point of PD initiation; point 2, 6-month point of PD; point 3, 3-month point prior to death or last follow-up) because we tried to identify correctable factors influencing survival according to elapsed time. We considered the first point as the baseline condition at starting PD and the second point as a short-term correctable condition after starting PD. The third point was considered as a condition which could suggest impending death or had implications for survival. The following laboratory parameters of serum were analyzed because these were evaluated monthly: hemoglobin, blood urea nitrogen (BUN), creatinine, albumin, calcium, and phosphorus. The patients who were taking angiotensin-converting enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), statin, low-dose aspirin, digoxin, or diuretics for >50% of the follow-up period were noted. In addition, we investigated whether PD patients were treated with an ACEI or ARB over the last 6 months before endpoints. We considered taking medication for >50% of the follow-up period and for >6 months before an endpoint as having a clinical effect on a patient’s outcome. All patients received erythropoietin injections for anemia correction.
Statistical Analysis
Data are presented as mean ± SD. The Fisher’s exact test was used to compare categorical data between two groups. Comparisons of parametric data between the groups with and without 3-year survival were analyzed with an independent t-test. The influential factors affecting survival were analyzed by Kaplan–Meier models using the log-rank test. Serum creatinine and albumin cutoff values for survival analysis were determined by area under the curve values through a receiver operating characteristic (ROC) curve. The Cox regression hazard model for multivariate analysis was applied to determine the factors independently associated with 3-year survival and total survival in PD patients. The variables for adjustments included age, gender, comorbid CVD (IHD, HF), albumin 2 (albumin level at 6-month PD point), albumin 3 and creatinine 3 (albumin and creatinine levels at 3-month point prior to death or last follow-up), and ACEI/ARB 2 (taking ACEI or ARB medicine during the last 6 months), which are significantly related to 3-year survival by univariate Cox regression analysis. p-Values of <0.05 were considered as significant. All statistical calculations were performed with SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline Characteristics at the Time of PD Initiation
The enrolled patients consisted of 57 men and 61 women, with a mean age of 54.6 ± 12.7 years (range: 19–82 years), survival rate of 65.3%, and PD duration of 47.1 ± 21.5 months. The baseline characteristics of the enrolled patients and prescribed medications are provided in . Of the enrolled patients, 55.9% had DM, 75.4% had HTN, 22.9% had IHD, and 13.6% had HF. Eighty-six patients (72.9%) were treated with ACEI/ARB for >50% of the follow-up period.
Table 1. Baseline characteristics at the time of PD initiation and prescribed medications for the PD duration.
Comparison of Clinical Findings According to 3-Year Survival
A comparison of patient characteristics and medications according to 3-year survival is summarized in . The 3-year survival rate was 74.6%. The 3-year survival group was younger (52.3 ± 12.7 vs. 61.4 ± 10.1 years, p = 0.001), male gender (55.7% vs. 26.7%, p = 0.006), and less likely to have IHD (19.3% vs. 40.7%, p = 0.024), or HF (8.5% vs. 34.6%, p = 0.003) than the 3-year nonsurvival group. In addition, the 3-year survival group had a higher serum albumin level at the 6-month point of PD than the 3-year nonsurvival group. The 3-year survival group had higher serum albumin and creatinine levels at the 3-month point prior to death or last follow-up than the 3-year nonsurvival group. The percentage of 3-year survival patients who showed a decreased serum albumin level compared with a baseline serum albumin level at the 6-month point of PD (39.8% vs. 63.3%, p = 0.034) and the 3-month point prior to death or last follow-up (38.6% vs. 61.4%, p = 0.021) was significantly lower than the 3-year nonsurvival patients. PD patients receiving ACEI or ARB treatment during the final 6-month endpoint tended to have longer survival than those not treated with ACEI or ARB.
Table 2. Comparisons of patient characteristics and medications according to 3-year survival.
Risk Factors Influencing Mortality
The most common cause of death was CVD (41.5%), including myocardial infarction or HF. The second most common cause of death was infection (29.3%), including sepsis in this retrospective study.
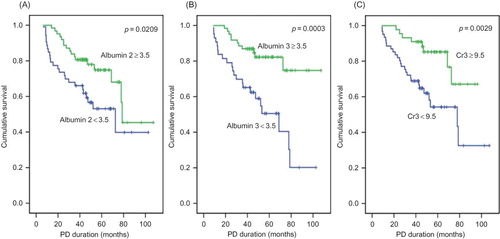
With respect to gender, the survival curve crossed after 3 years of follow-up; therefore, survival in males declined later. Patients without underlying IHD, HF, or with ACEI or ARB treatment during the last 6 months had significantly improved survival as determined by the Kaplan–Meier method (IHD, p = 0.0035; HF, p = 0.0014; and ACEI or ARB treatment, p = 0.0042) ( and ). PD patients were divided into two groups on the basis of a serum creatinine cutoff value of 9.5 mg/dL by ROC curve analysis with 76.5% sensitivity and 50.7% specificity. PD patients who had serum creatinine levels of >9.5 mg/dL at the 3-month point prior to death or last follow-up (creatinine 3) also had an improved survival curve (p = 0.0029) (). PD patients were also divided into two groups on the basis of serum albumin level (3.5 g/dL) by an ROC curve with 66.7% sensitivity and 70.4% specificity. PD patients who had a serum albumin level of >3.5 g/dL at the 6-month point of PD and at the 3-month point prior to death or last follow-up had improved survival curves (albumin 2, p = 0.0209; albumin 3, p = 0.0003) (). PD patients taking an ACEI or ARB for >50% of the follow-up period had favorable survival, but it was not statistically significant (p = 0.0505).
Figure 1. Cumulative survival curves for all-cause mortality according to gender (A), ischemic heart disease (IHD) (B), and heart failure (HF) (C).
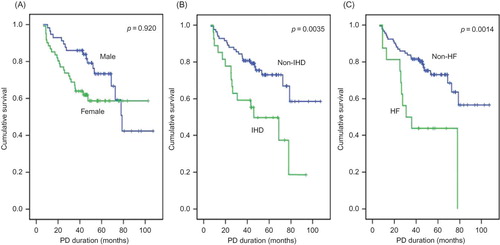
Table 3. Risk factors influencing mortality according to elapsed time by the multivariate Cox proportional hazard model.
HF was found to be an independent factor related to 3-year survival by the multivariate Cox regression model (). The serum albumin level at the 3-month point prior to death or last follow-up (albumin 3) was found to be an independent factor related to long-term survival (47.1 ± 21.5 months).
Figure 2. Cumulative survival curves for all-cause mortality according to angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) treatment. ACEI/ARB 1 reflects ACEI or ARB treatment during >50% of follow-up period (A). ACEI/ARB 2 reflects ACEI or ARB treatment during the last 6 months (B).
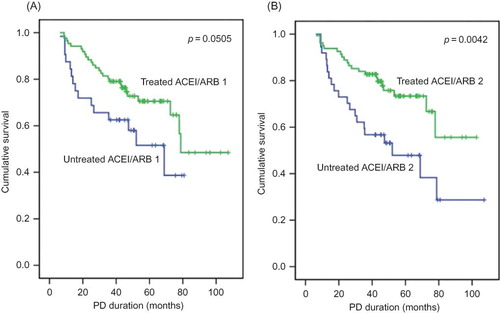
Figure 3. Cumulative survival curves for all-cause mortality according to albumin (A, B) and creatinine (Cr) (C). Creatinine 3 and albumin 3 indicate the creatinine and albumin levels at the 3-month point prior to death or last follow-up. Albumin 2 indicates the albumin levels at the 6-month PD point.
DISCUSSION
A recent study demonstrated that low serum albumin levels predicted mortality from all causes, including cardiovascular-related mortality and infection-related mortality in PD patients.Citation12 Especially, adjusted mortality from all causes was significantly lower in PD patients with a >0.3 g/dL increase in their serum albumin level over 6 months. Our present study also showed that a higher percentage of PD patients who survived >3 years had increased serum albumin levels at the 6-month point of PD compared with baseline serum albumin levels. It was also proved by our survival curves that an increased and maintained serum albumin level of >3.5 g/dL after the start of PD was an important factor for long-term survival. Jones et al.Citation13 also reported that a change in serum albumin offered better discrimination of PD outcome. Therefore, an increasing serum albumin level up to the 6-month point of PD is beneficial for improving 3-year survival. Furthermore, efforts for increasing and maintaining serum albumin levels at >3.5 g/dL according to elapsed time are important for long-term survival of PD patients.
The causes of hypoalbuminemia in PD patients are multifactorial. One common cause of hypoalbuminemia can be nutritional state.Citation14 It was considered that hypoalbuminemia resulted from poor oral intake, but serum albumin synthesis could also be decreased by the presence of inflammation. Peritoneal albumin loss is another cause of hypoalbuminemia in PD patients. Recent studies suggested that hypoalbuminemia reflects a volume overloaded state, and one independent predictor of whole body overhydration was low serum albumin levels in PD patients.Citation15,16 In this study, the authors demonstrated that the serum albumin level at the 3-month point prior to death or last follow-up was found to be an independent factor for survival in PD patients. Thus, it is necessary to solve problems such as malnutrition, inflammation, and volume overload that can affect serum albumin levels if the serum albumin level is decreasing after initiation of PD.
Sipahioglu et al.Citation15 reported that the relative risk of death as predicted by creatinine levels is 0.75 in Turkish PD patients. The serum creatinine level at the 3-month point prior to death or last follow-up was found to be a factor affecting survival of PD patients in our current study. The baseline serum creatinine level and serum creatinine level at the 6-month point of PD may reflect renal function, but the serum creatinine level at the 3-month point prior to death or last follow-up reflects renal function to a lesser degree. A high serum creatinine level is associated with muscle mass in PD patients with severely decreased renal function. A previous report has shown that an elevated serum creatinine is also related to a lean body mass, nutritional status, and dietary protein intake in dialysis patients.Citation9 Like albumin, a high serum creatinine level was related to favorable nutritional status. Thus, it is essential to consider nutritional status and muscle mass in patients with low and decreased serum creatinine levels undergoing PD for a longer period of time.
CVD is the main cause of death in dialysis patients. Similarly, CVD including HF was the leading cause of mortality in this study. HF was found to be an independent factor affecting the 3-year survival in our study. Wang et al.Citation17 reported that HF was a highly prevalent complication in long-term PD patients and predicted adverse clinical outcomes in 4-year prospective study. Fluid overload is a common problem in PD patients and may be associated with a higher prevalence of CVD. Therefore, an exact evaluation of volume status and adequate control of volume overload may improve survival in PD patients with HF. It should be noted that while HF was an independent factor affecting the 3-year survival rate, the albumin level at the 3-month point prior to death or last follow-up replaced HF as an independent factor for survival in this study. Uncontrolled volume status and HF deteriorate nutritional status, and then malnutrition again induces hypoalbuminemia, bringing about a vicious circle.Citation18,19 HF and a low albumin level are closely related to each other and further investigations are needed.
Several studies have been performed regarding the relationship of medication and dialysis mortality; representative medications are ACEI and ARB. Angiotensin II is a strong vasoconstrictor involved in myocardial fibrosis after myocardial infarction. ACEI and ARB are known to have blood pressure-lowering effects and cardiovascular protective effects, such as prevention of myocardial fibrosis and improvement in ventricular remodeling.Citation20 Dialysis patients have a 10 to 20-fold increased cardiovascular mortality compared with an age-matched and sex-matched population without renal disease; thus, the treatment with ACEI or ARB is considered for reducing cardiovascular mortality.Citation21,22 In this study, treatment with ACEI or ARB for longer periods or continuously taking ACEI or ARB for the last 6 months is the important factor affecting survival in PD patients, although it is not an independent factor for survival. In an experimental model in rats, ACEI and ARB use decreased peritoneal thickening and fibrosis, thus preventing the increase in mass transfer area coefficients.Citation23 ACEI and ARB treatments are expected to improve peritoneal function in PD patients.Citation24,25 These cardioprotective and peritoneal preserving effects of ACEI and ARB may be beneficial for survival of PD patients, but further investigations are needed.
Our study had several limitations, including its retrospective design. First, information for peritoneal transport rate and residual renal function including urine volume was not available. We did not investigate vascular calcification or new CVD events and parameters for nutrition and inflammation, except for albumin and creatinine, because we could not collect all three points data according to elapsed time. Despite these limitations, our study demonstrated that correctable serum albumin and creatinine levels according to elapsed time were important markers influencing the survival of PD patients.
In conclusion, a medical history of HF as an uncorrectable factor was useful information for predicting 3-year survival, and a low serum albumin level according to elapsed time should be corrected to improve survival in PD patients.
Declaration of interest: This study was supported by research funds from Dong-A University. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Fenton SS, Schaubel DE, Desmeules M, . Hemodialysis versus peritoneal dialysis: A comparison of adjusted mortality rates. Am J Kidney Dis. 1997;30:334–342.
- Serkes KD, Blagg CR, Nolph KD, Vonesh EF, Shapiro F. Comparison of patient and technique survival in continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis: A multicenter study. Perit Dial Int. 1990;10:15–19.
- Johnson DW, Dent H, Hawley CM, . Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin J Am Soc Nephrol. 2009;4:1620–1628.
- Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010;21:499–506.
- Oreopoulos DG, Ossareh S, Thodis E. Peritoneal dialysis: Past, present, and future. Iran J Kidney Dis. 2008;2:171–182.
- USRDS. The United States Renal Data System. Am J Kidney Dis. 2003;42:1–230.
- Han SH, Lee SC, Ahn SV, . Improving outcome of CAPD: Twenty-five years’ experience in a single Korean center. Perit Dial Int. 2007;27:432–440.
- Phelan PJ, O’Kelly P, Walshe JJ, Conlon PJ. The importance of serum albumin and phosphorous as predictors of mortality in ESRD patients. Ren Fail. 2008;30:423–429.
- Trivedi H, Tan SH, Prowant B, . Predictors of death in patients on peritoneal dialysis: The Missouri Peritoneal Dialysis Study. Am J Nephrol. 2005;25:466–473.
- Prasad N, Gupta A, Sinha A, . A comparison of outcomes between diabetic and nondiabetic CAPD patients in India. Perit Dial Int. 2008;28:468–476.
- Liao CT, Chen YM, Shiao CC, . Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant. 2009;24:2909–2914.
- Mehrotra R, Duong U, Jiwakanon S, . Serum albumin as a predictor of mortality in peritoneal dialysis: Comparisons with hemodialysis. Am J Kidney Dis. 2011;58:418–428.
- Jones CH, Newstead CG, Wills EJ, Davison AM. Serum albumin and survival in CAPD patients: The implications of concentration trends over time. Nephrol Dial Transplant. 1997;12:554–558.
- Enia G, Sicuso C, Alati G, Zoccali C. Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant. 1993;8:1094–1098.
- Sipahioglu MH, Aybal A, Unal A, Tokgoz B, Oymak O, Utas C. Patient and technique survival and factors affecting mortality on peritoneal dialysis in Turkey: 12 years’ experience in a single center. Perit Dial Int. 2008;28:238–245.
- John B, Tan BK, Dainty S, Spanel P, Smith D, Davies SJ. Plasma volume, albumin, and fluid status in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2010;5:1463–1470.
- Wang AY, Wang M, Lam CW, Chan IH, Lui SF, Sanderson JE. Heart failure in long-term peritoneal dialysis patients: A 4-year prospective analysis. Clin J Am Soc Nephrol. 2011;6:805–812.
- Don BR, Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437.
- Cheng LT, Tang W, Wang T. Strong association between volume status and nutritional status in peritoneal dialysis patients. Am J Kidney Dis. 2005;45:891–902.
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med. 2001;134:629–636.
- Fang W, Oreopoulos DG, Bargman JM. Use of ACE inhibitors or angiotensin receptor blockers and survival in patients on peritoneal dialysis. Nephrol Dial Transplant. 2008;23:3704–3710.
- Wetmore JB, Shireman TI. The ABCs of cardioprotection in dialysis patients: A systematic review. Am J Kidney Dis. 2009;53:457–466.
- Ersoy R, Celik A, Yilmaz O, . The effects of irbesartan and spironolactone in prevention of peritoneal fibrosis in rats. Perit Dial Int. 2007;27:424–431.
- Heaf JG, Sarac S, Afzal S. A high peritoneal large pore fluid flux causes hypoalbuminaemia and is a risk factor for death in peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20:2194–2201.
- Kolesnyk I, Dekker FW, Noordzij M, le Cessie S, Struijk DG, Krediet RT. Impact of ACE inhibitors and AII receptor blockers on peritoneal membrane transport characteristics in long-term peritoneal dialysis patients. Perit Dial Int. 2007;27:446–453.