Abstract
Background: Previous studies have demonstrated the role of inflammation in diabetic nephropathy (DN). Neutrophil to lymphocyte ratio (NLR) rather than other white cell parameters was found to be a useful inflammatory marker to predict adverse outcomes in medical and surgical conditions. Nevertheless, the value of NLR in predicting DN has not been elucidated. Method: An observational study included 338 diabetic patients, who were followed at our clinic between 2007 and 2009. We arranged our patients into tertiles according to their 2007 NLR. The primary outcome was continuous decrease of GFR >12 mL/min between 2007 and 2009 with the last GFR <60 mL/min. Result: The lowest NLR tertile had fewer patients (2.7%) with primary outcome (i.e., worsening renal function) compared with middle and highest NLR tertiles, which had more patients with primary outcomes (8.7% and 11.5%, respectively) with a significant p-value 0.0164. When other potential confounders were individually analyzed with NLR tertile, the NLR tertiles remained a significant predictor of poor GFR outcome in the presence of other variables (hemoglobin A1C, systolic blood pressure, diastolic blood pressure, age, and congestive heart failure with p-values 0.018, 0.019, 0.017, 0.033, and 0.022, respectively). Conclusion: NLR predicted the worsening of the renal function in diabetic patients. Further studies are needed to confirm this result.
INTRODUCTION
Diabetic nephropathy (DN) is the leading cause of renal failure in the United States.Citation1,2 Over the last 20 years, the prevalence of end-stage renal disease (ESRD) markedly declined among diabetes mellitus (DM) type I population to be 2–7%.Citation3–5 This decline is believed to be due to more diabetic care, which included the usage of hemoglobin A1C targeting treatment and availability of early markers for DN (e.g., microalbuminuria). Similar decline in the prevalence of ESRD in DM type II was also found over the last few decades.Citation6 Microalbuminuria was found to be one of the earliest markers for DN.Citation7 The reduction in microalbuminuria was associated with a slower decline in glomerular filtration rate (GFR) and decreased cardiovascular risk.Citation8,9 Later, the role of inflammation and inflammatory cytokines in DN was studied over the last few years.Citation10–16 Inflammation secondary to ischemia and endothelial dysfunction was found to be associated with the development of DN.Citation17–19 Some medications were found to slow down the progression of DN through suggested anti-inflammatory actions.Citation20–26 Neutrophil to lymphocyte ratio (NLR) rather than other white cell parameters (e.g., total white cell, monocyte count, and absolute neutrophil count) was found to be a useful inflammatory marker that predicts adverse outcomes in many medical and surgical conditions.Citation27–30 This finding was found not only in acute conditions but also in stable and preoperative medical conditions.Citation31–33 In a cross-sectional study, Chung et al.Citation34 found a higher neutrophil and lower lymphocyte count in patients with microalbuminuria and overt nephropathy compared with diabetics without albuminuria. Nevertheless, the usefulness of NLR as a predictor of worsening of renal functions among diabetic patients has not been elucidated.
METHODS
In a retrospective longitudinal study, our study population of nonselected 338 patients, who visited our diabetes clinic from January to December 2007, was followed up until December 2009. All included patients underwent complete physical exam and routine biochemical analysis [including complete blood count (CBC) and basic metabolic panel (BMP)] in 2007 and had at least one set of BMP on their 2008 and 2009 follow-up visits. We excluded patients with active cancer, hematological proliferative disorder, active or chronic inflammatory or autoimmune disease, and those who received steroids or any other immunosuppressive therapy or chemotherapy just before or during the study period. Before the commencement of the study, the primary end point was set to be a continuous dropping of the estimated GFR (eGFR) more than 12 mL/min over the 3-year follow-up period, with the last eGFR <60 mL/min. Each included patient’s chart was reviewed by two independent physicians. The hematological variables [total white blood cell (WBC), neutrophil, lymphocyte, and monocyte absolute counts, hemoglobin, hemoglobin A1C] were obtained by using Coulter counter® technique (Coulter Gen.S Hematology Analyzer, Beckman Coulter Corp., Hialeah, FL, USA). The GFR was estimated with the 4-variable Modification of Diet in Renal Disease (MDRD) equation using serum creatinine, age, race, and gender. Positive smoking status included both current and prior smokers. The study was approved by the Institutional Review Board at Staten Island University Hospital, New York.
Statistical Analysis
Patients were divided into three equal groups (tertile model) according to their NLR in 2007 (cutoff points were the 33rd and 66th NLR values). The distributions of continuous and categorical variables were presented as means, standard deviations, frequencies, and percentages. Group comparisons used χ2 analysis for categorical data. Continuous variables were compared with analysis of variance or Kruskal–Wallis analysis of ranks, depending on the probability distribution of the variable. All probabilities were two sided and p-values <0.05 were considered statistically significant. The exact two-tailed Cochran–Armitage test for trend was used to test for an association between NLR tertile and the incidence of a poor GFR outcome. A univariate screen of all potential predictors of poor GFR outcome was performed. Due to the small number of poor outcomes (n = 26), a logistic model would not support more than one covariate in addition to NLR tertile. Accordingly, a modification to this multivariable approach was taken whereby several logistic models were constructed, corresponding to the NLR tertile and each of the above-mentioned potential confounders individually. The objective was to see whether the effect of tertile was changed when adjusting for a given covariate. All data were analyzed using the Statistical Analysis System, version 9.2 (SAS® Institute, Cary, NC, USA).
RESULTS
The Baseline Characteristics
The patients were arranged into three equal groups according to their baseline 2007 NLR values [first tertile (NLR < 1.6) = 110 patients, second tertile (NLR 1.6–2.36) = 115 patients, and third tertile (NLR > 2.36) = 113 patients]. There were significantly higher frequencies of male and Caucasian patients in the third (highest NLR) tertile compared with the lower NLR tertile(). Additionally, the patients in the higher NLR tertile were significantly older with higher average body mass index and higher frequencies of coronary artery disease and chronic lung disease compared with the lowest NLR tertile. Likewise, there was a trend of more patients with congestive heart failure in the higher NLR tertile. The rates of treatment with aspirin, nonsteroidal analgesics, clopidogrel, statin, angiotensin-converting enzymes inhibitor, and angiotensin receptor blockers were nonstatistically different among the three NLR tertile groups. There was no difference in baseline systolic blood pressure, diastolic blood pressure, and hemoglobin A1C among the three NLR tertiles.
Table 1. Baseline characteristics of our diabetic population by initial (2007) neutrophil to lymphocyte ratios (NLRs).
NLR as a Predictor of the Primary Outcome
Patients in the higher NLR tertile had significantly higher baseline serum creatinine and lower eGFR compared with lower NLR tertile ( 2). The lowest NLR tertile had fewer patients (2.7%) with primary outcome (i.e., dropping GFR >12 mL/min/3 years) compared with middle and highest NLR tertiles, which had more patients with primary outcomes (8.7% and 11.5%, respectively) with a significant p-value = 0.0164 according to the exact two-tailed Cochran–Armitage test (). When each of the potential confounders was individually analyzed with the NLR tertile, the NLR tertile remained a significant predictor of poor GFR outcome in the presence of other variables (hemoglobin A1C, systolic blood pressure, diastolic blood pressure, age, and congestive heart failure with p-values 0.018, 0.019, 0.017, 0.033, and 0.022, respectively). When the urine albumin over creatinine ratio (UACR) was considered, the NLR showed a trend of poor outcome in the highest versus the lowest NLR tertiles (p = 0.055) and the UACR was a significant predictor of poor renal outcome (). Additionally, there was a significantly higher prevalence of chronic kidney disease (CKD) both in 2007 and 2009 in the highest NLR tertile compared with the lower tertiles (). Further subanalysis demonstrated a higher primary outcome in the lowest lymphocyte tertile compared with the highest tertile (p = 0.01). However, the highest neutrophil tertile had a higher nonsignificant primary outcome compared with the lowest neutrophil tertile (p = 0.09).
Figure 1. Primary outcome (i.e., dropping of GFR >12 mL/min/3 years) according to initial (2007) neutrophil to lymphocyte ratio tertiles; p = 0.01 according to the two-tailed Cochran–Armitage test.
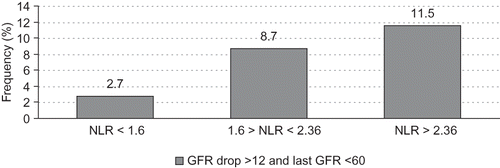
Figure 2. Prevalence of chronic kidney disease (CKD) according to initial (2007) neutrophil to lymphocyte ratio tertiles.
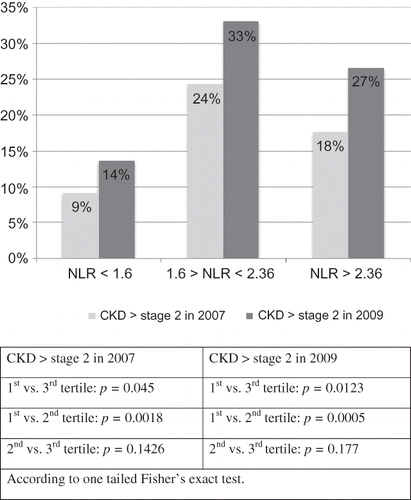
Table 2. Baseline characteristics of our diabetic population by initial (2007) neutrophil to lymphocyte ratios (NLRs).
Table 3. The predictive value of NLR adjusted to other confounding variables.
DISCUSSION
The Main Finding
The association between an increased neutrophil count and type II diabetes, insulin resistance, and DN was already described in the literature.Citation34 The novel finding in our study was that NLR as an inflammatory marker is an independent significant predictor of the worsening of the renal function in diabetic patients, even after adjusting for possible confounders such as age, blood pressure, glucose control, and congestive heart failure.
Superiority of NLR to Other Leukocyte Parameters
The NLR as a marker is superior to other leukocyte parameters (e.g., neutrophil, lymphocyte, and total leukocyte counts) due to the stability of NLR compared with the absolute counts that could be altered by various physiological, pathological, and physical factors. Moreover, NLR represents a combination of two markers of uncontrolled chronic inflammatory condition (i.e., high neutrophil and low lymphocyte). Prior studies demonstrated this superiority of NLR to the total leukocyte countCitation31,35 and to individual neutrophil and lymphocyte countsCitation31,36 in predicting adverse outcomes in cardiovascular diseases. In our study, the neutrophil and lymphocyte tertile-adjusted models were not significantly associated with worsening renal functions compared with the NLR model.
Neutrophils, Lymphocytes, Inflammation, and CKD
We postulate that a high neutrophil count is a marker of ongoing destructive nonspecific inflammatory process and a low lymphocyte count is a marker of inadequate regulatory and quiescent immunity pathway. Rapid progression of DN in particular has been shown to be linked to inflammation and episodes of acute renal failure.Citation37 Prominent clusters of inflammatory cells were detected in the renal biopsy specimens.Citation37 Post-ischemic inflammatory syndrome that accelerates diabetic CKD is a critical determinant of injury and can be successfully treated.Citation38 In cancer, neutrophil-derived reactive oxygen species release and subsequent events lead to enhanced angiogenesis.Citation39,40 A similar sequence of events may be responsible for the development of the abnormal angiogenesis that was described in DN as a microvascular complication of DM.Citation41,42 The increased spontaneous adherence of neutrophil to endothelial cells was also described as a possible mechanism of DN and proteinuria.Citation43 Moreover, studies demonstrated the usefulness of neutrophil gelatinase-associated lipocalin (NGAL) as a significant marker of DN and CKD progression.Citation44,45 Interestingly, myeloperoxidase (MPO), which is secreted during activation of neutrophils, may serve as one mechanistic link among persistent inflammation, oxidative stress, and cardiovascular disease. Statin use (p < 0.01) was significantly associated with a decreased MPO activity.Citation46 On the other hand, Chung et al.Citation34 demonstrated the association between low lymphocyte count and the severity of nephropathy. Likewise, lymphopenia was associated with adverse outcomes among coronary artery disease and congestive heart failure patients.Citation47,48
NLR (Marker, Factor, or a Predictor)
While Chung et al.Citation34 reported the association between leukocytosis, low lymphocyte count, and DN in their cross-sectional study, we are reporting not only the higher prevalence of CKD with high NLR but also the worsening of renal function over the follow-up period. We suggest that NLR is not only a marker of presence of nephropathy but a factor in the pathogenesis of the disease. However, we cannot validate the causality of high NLR to this worsening of renal function, which warrants further studies.
Limitations
Although we prospectively followed our study population, the patients were retrospectively recruited from our database. The lack of ethnic diversity in this single-center study limits the generalizability of our findings to other populations. We used the 4-variable MDRD study equation to estimate GFR, which may be imprecise, particularly in the normal renal function range; nonetheless, we used it to assess eGFR changes in the same participants and not as a screening tool for CKD. Finally, the study lacks data on other inflammatory markers (ESR, C-reactive protein, others).
CONCLUSIONS
Higher NLR is a significant predictor of higher short- and long-term all-cause mortality in multiple conditions, and it seems to have the same importance in the prediction of worsening renal function in diabetics. Further prospective, multicenter studies are needed to verify this result, and then, if validated, the reasons for this association of the NLR with CKD in the diabetic population need to be studied at the molecular level. Is the NLR a marker of an etiologic agent in renal disease progression? Can altering the NLR change the prognosis? A proteomic discovery of DN biomarkers that may correlate with higher NLR may be another application to our finding.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Krolewski M, Eggers PW, Warram JH. Magnitude of end-stage renal disease in IDDM: A 35 year follow-up study. Kidney Int. 1996;50(6):2041–2046.
- Matsushima M, Tajima N, LaPorte RE, . Markedly increased renal disease mortality and incidence of renal replacement therapy among IDDM patients in Japan in contrast to Allegheny County, Pennsylvania, USA. Diabetes Epidemiology Research International (DERI) US-Japan Mortality Study Group. Diabetologia. 1995;38(2):236–243.
- Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med. 1994;330(1):15–18.
- Costacou T, Ellis D, Fried L, Orchard TJ. Sequence of progression of albuminuria and decreased GFR in persons with type 1 diabetes: A cohort study. Am J Kidney Dis. 2007;50(5):721–732.
- Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. J Am Med Assoc. 2005;294(14):1782–1787.
- Pavkov ME, Knowler WC, Bennett PH, Looker HC, Krakoff J, Nelson RG. Increasing incidence of proteinuria and declining incidence of end-stage renal disease in diabetic Pima Indians. Kidney Int. 2006;70(10):1840–1846.
- Eknoyan G, Hostetter T, Bakris GL, . Proteinuria and other markers of chronic kidney disease: A position statement of the National Kidney Foundation (NKF) and the National Institute of Diabetes and Digestive and Kidney diseases (NIDDK). Am J Kidney Dis. 2003;42(4):617–622.
- Araki S, Haneda M, Koya D, . Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes. 2007;56(6):1727–1730.
- Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant. 2004;19(11):2784–2788.
- Choudhary N, Ahlawat RS. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: New evidence linking inflammation, glycemic control, and microalbuminuria. Iran J Kidney Dis. 2008;2(2):72–79.
- Kuhad A, Sachdeva AK, Chopra K. Attenuation of renoinflammatory cascade in experimental model of diabetic nephropathy by sesamol. J Agric Food Chem. 2009;57(14):6123–6128.
- Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19(3):433–442.
- Rivero A, Mora C, Muros M, Garcia J, Herrera H, Navarro-Gonzalez JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond). 2009;116(6):479–492.
- Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol. 2008;93(7):817–824.
- Lewis A, Steadman R, Manley P, . Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histol Histopathol. 2008;23(6):731–739.
- Wang JJ, Zhang SX, Mott R, . Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294(5):F1166–F1173.
- Astrup AS, Tarnow L, Pietraszek L, . Markers of endothelial dysfunction and inflammation in type 1 diabetic patients with or without diabetic nephropathy followed for 10 years: Association with mortality and decline of glomerular filtration rate. Diabetes Care. 2008;31(6):1170–1176.
- Kelly KJ, Burford JL, Dominguez JH. Postischemic inflammatory syndrome: A critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297(4):F923–F931.
- Persson F, Rossing P, Hovind P, . Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scand J Clin Lab Invest. 2008;68(8):731–738.
- Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY, Lim SJ. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am J Nephrol. 2009;29(3):274–282.
- Ko GJ, Kang YS, Han SY, . Pioglitazone attenuates diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. Nephrol Dial Transplant. 2008;23(9):2750–2760.
- Navarro-Gonzalez JF, Jarque A, Muros M, Mora C, Garcia J. Tumor necrosis factor-alpha as a therapeutic target for diabetic nephropathy. Cytokine Growth Factor Rev. 2009;20(2):165–173.
- Ogawa S, Kobori H, Ohashi N, . Angiotensin II type 1 receptor blockers reduce urinary angiotensinogen excretion and the levels of urinary markers of oxidative stress and inflammation in patients with type 2 diabetic nephropathy. Biomark Insights. 2009;4:97–102.
- Goicoechea M, de Vinuesa SG, Verdalles U, . Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393.
- Pergola PE, Krauth M, Huff JW, . Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b-4 CKD. Am J Nephrol. 2011;33(5):469–476.
- Pergola PE, Raskin P, Toto RD, . Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336.
- Goodman DA, Goodman CB, Monk JS. Use of the neutrophil: Lymphocyte ratio in the diagnosis of appendicitis. Am Surg. 1995;61(3):257–259.
- Gurm HS, Bhatt DL, Lincoff AM, . Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: Insights from the EPIC, EPILOG, and EPISTENT trials. Heart. 2003;89(10): 1200–1204.
- Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–657.
- Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14.
- Gibson PH, Croal BL, Cuthbertson BH, . Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154(5):995–1002.
- Gillum RF, Mussolino ME, Madans JH. Counts of neutrophils, lymphocytes, and monocytes, cause-specific mortality and coronary heart disease: The NHANES-I epidemiologic follow-up study. Ann Epidemiol. 2005;15(4):266–271.
- Tsai JC, Sheu SH, Chiu HC, . Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2007;23(2):111–118.
- Chung FM, Tsai JC, Chang DM, Shin SJ, Lee YJ. Peripheral total and differential leukocyte count in diabetic nephropathy: The relationship of plasma leptin to leukocytosis. Diabetes Care. 2005;28(7):1710–1717.
- Nunez J, Nunez E, Bodi V, . Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101(6):747–752.
- Azab B, Zaher M, Weiserbs KF, . Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106(4):470–476.
- Kelly KJ, Dominguez JH. Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol. 2010;32(5):469–475.
- Kelly KJ, Dominguez JH. Treatment of the post-ischemic inflammatory syndrome of diabetic nephropathy. Nephrol Dial Transplant. 2010;25(10):3204–3212.
- De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10(15):4895–4900.
- Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: Potential implications for tumor progression. Cancer Res. 2005;65(19):8896–8904.
- Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am J Physiol Renal Physiol. 2011;302(3):F308–F315.
- Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58(7):1471–1478.
- Takahashi T, Hato F, Yamane T, . Increased spontaneous adherence of neutrophils from type 2 diabetic patients with overt proteinuria: Possible role of the progression of diabetic nephropathy. Diabetes Care. 2000;23(3):417–418.
- Bolignano D, Lacquaniti A, Coppolino G, . Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337–344.
- Bolignano D, Lacquaniti A, Coppolino G, . Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res. 2009;32(2):91–98.
- Stenvinkel P, Rodriguez-Ayala E, Massy ZA, . Statin treatment and diabetes affect myeloperoxidase activity in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2006;1(2):281–287.
- Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79(6):812–814.
- Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19–22.