Abstract
Kidney function is routinely monitored utilizing classic biochemical parameters including serum or plasma creatinine (Cr), and blood urea nitrogen (BUN) concentrations. This study demonstrates that the simultaneous assessment of plasma glutathione peroxidase (pGPx) and Cr levels provides a better strategy for the immediate follow-up of kidney function in organ recipients. Kidney recipients (Krs; n = 22) were recruited. Blood sampling schedule commenced at day 1 (pre-transplantation) and post-transplantation days (i.e., everyday from 1 until day 14, and thereafter on days 21, 28, 35, 42, 49, and 56). pGPx was measured spectrophotometrically. Candidates for transplantation exhibited lower pGPx than control subjects (42 ± 24 vs. 143 ± 31 U/L; p < 0.005). In Krs with a stable post-transplant outcome, pGPx increased to a maximum at day 28 (214 ± 61 U/L). In a Kr diagnosed with acute tubulonecrosis, pGPx provided a better predictive value (threefold increase) than Cr. In a Kr diagnosed with acute rejection, the increment in Cr values was found to be more pronounced than in pGPx values. The pGPx test is simple, inexpensive and automatable, and should be a valuable diagnostic tool of kidney function in organ recipients with and without troublesome outcome for the follow-up during hospitalization period.
INTRODUCTION
Kidney transplantation is a selected treatment for patients with end-stage renal disease (ESRD).Citation1 It has been recognized that renal recipients from living donors have longer survival rates than in those from cadaveric donors.Citation2,3 However, the underlying mechanism is not well established.Citation4–7 Kidney function is routinely monitored utilizing classic biochemical limits including serum or plasma creatinine (Cr), and blood urea nitrogen (BUN) concentrations.
Glutathione peroxidase (GPx) is a family of selenoproteins.Citation8,9 GPx exists in various isoenzymes including cytosolic (cGPx), gastrointestinal (GI-GPx), plasma (pGPx) and phospholipid hydroperoxide (PHGPx).Citation10 With the exception of PHGPx, which is a monomer, all other GPx isoenzymes consist of four identical subunits. Each subunit contains a single selene-cysteine residue that is essential for the catalytic activity of GPx.Citation11,12 GPx catalyzes the reduction of organic hydroperoxides (ROOH) and hydrogen peroxide (H2O2) utilizing glutathione as a reducing agent. Thus, one of the physiological functions of GPx is the protection of extracellular fluid components and cell surfaces against peroxide-mediated damage.Citation11–13
In the kidney, pGPx is highly abundant in the S1 and S2 segments of the proximal tubules.Citation14,15 Lower pGPx activity (39–52%) has been reported in nondialyzed individuals with renal failureCitation16–19 and those either on hemodialysisCitation18,20,21 or on Continuous Ambulatory Peritoneal Dialysis (CAPD)Citation21,22 when compared with control subjects. The decline in pGPx activity in subjects with renal failure has been linked to the impairment of pGPx biosynthesis in the kidney.Citation19 Whitin et al.Citation23 analyzed pGPx activities in kidney recipients (Krs) subdivided according to organ source [i.e., living related donors (LRD) or cadaveric donors (CD)]. It was found that pGPx activities in Krs were improved rapidly within 1 week after organ replacement therapy. Two to three weeks post-transplantation, Krs exhibited higher pGPx activity than healthy control subjects. The trends for the improvement in pGPx activities post-surgery were similar when comparing organs from LRD or CD. Similarly, Zachara et al.Citation24 reported that pGPx activity was markedly improved immediately after transplantation. However, no difference was seen in the magnitude of pGPx activities between Krs and healthy controls.Citation25 These data indicate that monitoring pGPx activity in Krs may provide useful tool in detecting chronic allograft nephropathy (CAN), which is a leading cause of organ failure during the first year after renal transplantation.Citation26,27
The aims of this cross-sectional study were to explore the relationships between pGPx activities and the concentrations of serum Cr in organ recipients with and without adverse outcome. This study extends beyond previous investigations in the term that a homogenous group of Krs has been enrolled and that tighter measurement has been conducted during hospitalization period.
MATERIALS AND METHODS
Reagents
Reduced glutathione (GSH), glutathione reductase (GR), and nicotine amid adenine dinucleotide phosphate (NADPH) were purchased from Sigma Chemical Corporation (Dorset, UK). Tris-HCl buffer, NaN3, ethylenediaminetetraacetic acid (EDTA) and H2O2 were from E. Merck (Darmstadt, Germany). Kits for routine biochemical parameters were obtained from Pars Azmon Company (Tehran, Iran).
Studied Population
The recipients of first kidney (age range: 30–60 years, n = 22) were recruited through the Department of Nephrology, Immam Khomeini Teaching Hospital, Urmia University of Medical Sciences, Urmia, Iran. This study was commenced at April 2005 and terminated at September 2007. Inclusion criteria were males of age 30–60 years and recipients of first kidney. Exclusion criteria were diabetes, autoimmune disease, acute phase and/or chronic inflammation, and antioxidant oxidant supplementation 2 months pre-admission. The participants received a cocktail comprising Cyclosporine (5 mg/kg/day), Azathioprine (1 mg/kg/day), and Prednisolone (1 mg/kg/day) as immunosuppressive therapy (IT). For comparison, volunteer subjects (age and sex matched; n = 25) with no history of hypertension, diabetes, or renal disease were employed as the control group. Candidates for kidney transplantation gave their written consent after explaining the study objectives. This study was approved by the ethical committee at Urmia University of Medical Sciences.
Blood Collection and Plasma Preparation
For routine biochemical measurement, morning blood sample (2 mL) was collected before IT intake. The sample was allowed to coagulate at room temperature for 30 min and serum was obtained by centrifugation at 2000 × g for 10 min.
At the time of venipuncture for routine biochemical analyses, blood samples (2 mL) were also collected for measurement of pGPx activity. The sample was transferred into glass tube containing EDTA as an anticoagulant at a final concentration of 1 mg/mL. Sampling schedule commenced at day 1 (pre-transplantation) and post-transplantation days (i.e., everyday from 1 until day 14 and thereafter on days 21, 28, 35, 42, 49, and 56). Plasma was obtained by centrifugation at 2000 × g for 10 min at 4°C. Aliquots (200 μL) were transferred into Eppendorf tubes and subsequently stored at −80°C until analysis.
In the case of control group, blood samples were drawn after an overnight fast. The remaining procedures for blood separation were as described above.
Routine Biochemical Measurements
Glucose was measured using the hexokinase method. Uric acid was determined using a uricase-based assay. Creatinine was assessed employing a creatininase-based test. Total cholesterol was measured using the Cholesterol-C high-performance CHOD-PAP assay. Triacylglycerols was determined using the enzymatic method. Inorganic phosphor and calcium were measured spectrophotometrically. Glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) activities were monitored by coupling transaminase reactions. A continuous-monitoring technique based on the molar absorptivity of p-nitrophenol was used for the determination of alkaline phosphatase (ALP) activity. Sodium and potassium were determined flame photometrically. Urea was measured using colorimetric method by employing diacetyl monoxime.
Measurement of GPx Activity
pGPx activity was measured using coupled enzymatic reaction at 25°C as was previously described by Paglia and Valentine.Citation28 Briefly, coupling reaction was prepared in Tris-HCl buffer (50 mM; pH 7.6) comprising GSH, NADPH, EDTA, NaN3 and GR, at a final concentration of 1 mM, 0.2 mM, 2 mM, 1 mM and 100 units, respectively. The reaction was initiated by adding H2O2 at a final concentration (1 mM) and monitored for 3 min at 340 nm. One unit of the enzyme activity was expressed as 1 μmol of NADPH oxidized per minute, and the result was expressed as unit per liter of plasma.
Statistical Analysis
Analyses were performed using the SPSS software package for windows (version 13; SPSS, Chicago, IL, USA). Continuous data were expressed by the arithmetical mean ± SD. Paired t-test was used for comparison of the data from different sampling points. Differences between groups were analyzed using the t-test for two independent samples. Pearson’s correlation analysis was used to assess associations between parameters. A p-value <0.05 was considered statistically significant.
RESULTS
Clinical and biochemical characteristics of Krs (i.e., at pre-operation day and at days 1, 3, 7, and 14 post-operation) are presented in . As can be seen in , body weight and systolic blood pressure were slightly lower on day 14 post-operation when compared with pre-operation day. Diastolic blood pressure was also found to be lower but the difference did not reach statistical significance. The Krs exhibited lower levels of Cr, BUN, potassium, and phosphor (p < 0.05) when compared with baseline. Similarly, a decline in ALP activity was seen post-transplantation but the differences failed to reach statistical significance. On the contrary, a slight enhancement in cholesterol and TG contents was noticed after surgery when compared with baseline. A marginal increase in GPT activity was also detected after surgery.
Table 1. The profile for pGPx activity in Krs with stable post-operative outcome (n = 20).
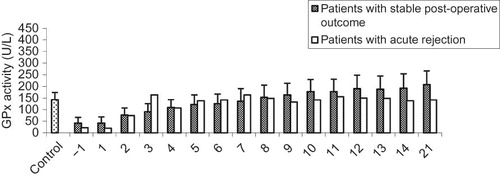
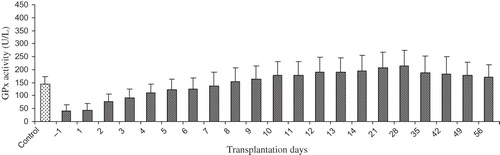
shows the alteration in pGPx activity in Krs with stable post-operation outcome. The mean value for pGPx activity among the candidates for kidney transplantation was markedly lower than among the control subjects (41.9 ± 24.2 vs. 143.35 ± 30.8 U/L; p < 0.005). No differences were observed in pGPx activity between the first day of pre-operation and the first day post-operation. However, significant elevations in pGPx activity were noticed from the second day onwards. pGPx activity reached a maximum on day 28 post-operation (214.2 ± 61.0 U/L). A 12% drop in pGPx activity was noted on day 35 when compared with day 28 (188.6 ± 63.2 vs. 214.2 ± 61.0 U/L; p > 0.05). From day 35 onwards, pGPx activity remained fairly unchanged.
Figure 1. The profile for pGPx activity in kidney recipient with stable post-operative outcome (n = 20). Sample collection was commenced at day 1 pre-transplantation and terminated at day 58 post-transplantation. Data are presented as mean ± SD.
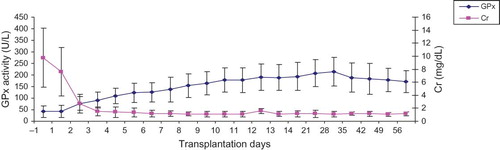
shows the concomitant changes in pGPx activity and Cr levels in Krs with stable post-operative outcome. Negative correlations were seen between GPx activity and Cr levels (rp = −0.753; p < 0.005).
Figure 2. The relationship between pGPx activity and Cr levels in Krs with stable post-operative outcome (n = 20). Sample collection was commenced at day 1 pre-transplantation and terminated at day 58 post-transplantation. Data are presented as mean ± SD.
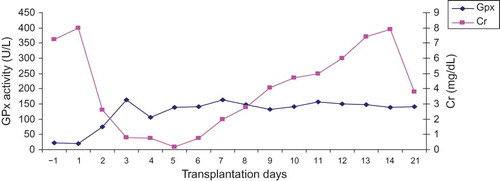
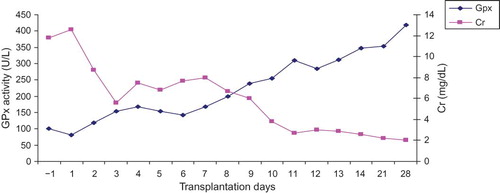
The pattern for pGPx activity for a kidney recipient diagnosed with acute tubulonecrosis (ATN) is shown in . The increments in pGPx up to day 9 post-surgery were similar to those with stable post-operative outcome. Thereafter, a marked enhancement in pGPx activity was observed on those with stable post-operative outcome. Measurement of pGPx activity was terminated on day 28 after surgery because the patient had to be moved to another ward. The relationship between pGPx activity and the levels of Cr is demonstrated in .
Figure 3. The profile for pGPx activity in kidney recipient with troublesome post-operative outcome (i.e., acute tubulonecrosis). Sample collection was commenced at day 1 pre-transplantation and terminated at day 28 post-transplantation.
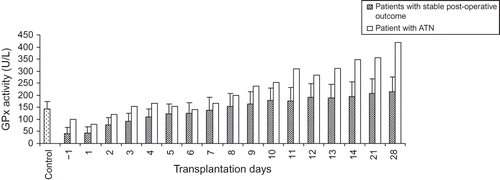
Figure 4. The relationship between pGPx activity and Cr levels in kidney recipient with troublesome post-operative outcome (i.e., acute tubulonecrosis). Sample collection was commenced at day 1 pre-transplantation and terminated at day 28 post-transplantation.
The trend for pGPx activity in a kidney recipient experiencing an episode of acute rejection (AR) is displayed in . pGPx activity approached the expected values for those with stable post-operative outcome (i.e., 164 U/L) at day 7 post-operation. pGPx activity subsequently dropped to the expected values for control group (143 U/L ± 30 U/L) and remained unchanged during the course of the investigation (i.e., 21 days). The association between pGPx activity and Cr concentrations is shown in .
DISCUSSION
Kidney replacement is the ultimate therapy for ESRD patients. Therefore, the diagnostic challenge is to detect early onset of renal dysfunction. Early diagnoses of renal dysfunction also imply better patient’s outcomes assuming the effective therapeutic regimen are utilized. Although biopsy is invasive, it remains as the golden standard method for the evaluation of renal dysfunction. In this investigation, pGPx activity has been monitored as an index of kidney function in conjugation with Cr and BUN in renal recipients. From a practical point of view, the pGPx assay is simple, inexpensive, and automatable.
We report that the mean pGPx activity in the candidate for renal transplantation was 29% that in the control group. This value is in good agreement with those (31%) obtained by Zachara et al.Citation24 Respective value reported by Whitin et al.Citation23 was 50% employing t-butyl hydroperoxide as substrate in the pGPx assay instead of hydrogen peroxide.
This study has revealed that the largest increment in pGPx activity in renal recipients from living donors with stable post-operative outcome occurred between day 1 and day 2 post-transplantation. The mean pGPx activity approached that of healthy control subjects within 9 days after transplantation. Maximum activity was recorded at day 28 post-surgery. These findings are in agreement with those previously reported by other investigators.Citation23,24
Additional support for the notion that the measurement of pGPx activity is a useful tool in detecting renal dysfunction is derived from examining the profile of pGPx activity in a kidney recipient experiencing an episode of ATN. As illustrated in , kidney recipient with ATN exhibited higher pGPx activities than those with stable post-operative outcome. On day 28 post-surgery, the value for pGPx in patients was about threefold higher than that in healthy control group. This may imply a greater demand on the kidney for increasing pGPx biosynthesis to maintain a normal renal function. On the contrary, the values for Cr on day 28 post-surgery revealed a marginal increase when compared with normal range and thus suggesting the importance of the simultaneous determination of pGPx and Cr as biomarkers of renal function in organ recipients.
The profiles for pGPx activity and Cr levels in a kidney recipient experiencing an episode of AR provide another proof for the usefulness of the inclusion of pGPx activity assay as complement for Cr during evaluation of kidney function post-surgery ( and ). The assessment of pGPx activity assay revealed that the trend of profile was similar to that of renal recipients with stable post-operative outcome albeit the magnitude of the activity was marginally attenuated. On the other hand, the assessment of Cr seemed to provide better diagnostic values of renal function.
Simic-ogrizovic et al.Citation29 explored the usefulness of assay pGPX activity in long-survival graft without and with dysfunction 5 years after transplantation. They reported that the mean of pGPx activity in Krs with stable post-operative outcome was similar to that of the healthy control group. On the contrary, a subgroup with chronic rejection exhibited lower pGPx activity (25%) compared with the value for healthy control subjects. Taken together, these findings imply that the simultaneous assessment of Cr and pGPx activity should serve as a valuable tool for the follow-up of renal function after renal transplantation and/or during surveys of long-survival graft.
In this cross-sectional investigation, we have shown that the inclusion of pGPx assay as a clinical biomarker should improve detection of renal malfunction in organ recipients during hospitalization period as well as in surveys of long-survival graft. Further studies are needed to establish the power of the pGPx assay in distinguishing between individuals with adverse outcome from those with stable outcome.
ACKNOWLEDGMENT
This project was supported by a grant from the deputy of research, Urmia University of Medical Sciences, Urmia, Iran.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Cameron JI, Whiteside C, Katz J, Devins GM. Differences in quality of life across renal replacement therapies: A meta-analytic comparison. Am J Kidney Dis. 2000;35:629–637.
- Cecka JM. Outcome statistics of renal transplants with an emphasis on long-term survival. Clin Transplant. 1994;8:324–327.
- Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333–336.
- Koo DD, Welsh KI, McLaren AJ, . Cadaver versus living donor kidneys: Impact of donor factors on antigen induction before transplantation. Kidney Int. 1999;56:1551–1559.
- Nijboer WN, Schuurs TA, van der Hoeven JA, . Effects of brain death on stress and inflammatory response in the human donor kidney. Transplant Proc. 2005;37:367–369.
- Pratschke J, Wilhelm MJ, Kusaka M, . Accelerated rejection of renal allografts from brain-dead donors. Ann Surg. 2000;232:263–271.
- Takada M, Nadeau KC, Hancock WW, . Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation. 1998;65:1533–1542.
- Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387:1329–1335.
- Brigelius-Flohe R, Flohe L. Is there a role of glutathione peroxidases in signaling and differentiation? Biofactors. 2003;17:93–102.
- Brown KM, Pickard K, Nicol F, . Effects of organic and inorganic selenium supplementation on selenoenzyme activity in blood lymphocytes, granulocytes, platelets and erythrocytes. Clin Sci (Lond). 2000;98:593–599.
- Forstrom JW, Zakowski JJ, Tappel AL. Identification of the catalytic site of rat liver glutathione peroxidase as selenocysteine. Biochemistry. 1998;17:2639–2644.
- Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta. 1985;839:62–70.
- Yamamoto Y, Takahashi K. Glutathione peroxidase isolated from plasma reduces phospholipid hydroperoxides. Arch Biochem Biophys. 1993;305:541–545.
- Avissar N, Ornt DB, Yagil Y, . Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol. 1994;266:C367–C375.
- Maser RL, Magenheimer BS, Calvet JP. Mouse plasma glutathione peroxidise. cDNA sequence analysis and renal proximal tubular expression and secretion. J Biol Chem. 1994;269:27066–27073.
- Bulucu F, Vural A, Aydin A, Sayal A. Oxidative stress status in adults with nephrotic syndrome. Clin Nephrol. 2000;53:169–173.
- Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, . Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med. 1996;21:845–853.
- Richard MJ, Arnaud J, Jurkovitz C, . Trace elements and lipid peroxidation abnormalities in patients with chronic renal failure. Nephron. 1991;57:10–15.
- Schiavon R, Guidi GC, Biasioli S, De Fanti E, Targa L. Plasma glutathione peroxidase activity as an index of renal function. Eur J Clin Chem Clin Biochem. 1994;32:759–765.
- Roxborough HE, Mercer C, McMaster D, Maxwell AP, Young IS. Plasma glutathione peroxidase activity is reduced in hemodialysis patients. Nephron. 1999;81:278–283.
- Yoshimura S, Suemizu H, Nomoto Y, . Plasma glutathione peroxidase deficiency caused by renal dysfunction. Nephron. 1996;73:207–211.
- Martin-Mateo MC, del Canto-Jafiez E, Barrero-Martinez MJ. Oxidative stress and enzyme activity in ambulatory renal patients undergoing continuous peritoneal dialysis. Ren Fail. 1998;20:117–124.
- Whitin JC, Tham DM, Bhamre S, . Plasma glutathione peroxidase and its relationship to renal proximal tubule function. Mol Genet Metab. 1998;65:238–245.
- Zachara BA, Wlodarczyk Z, Masztalerz M, . Selenium concentrations and glutathione peroxidase activities in blood of patients before and after allogenic kidney transplantation. Biol Trace Elem Res. 2004;97:1–13.
- Zachara BA, Gromadzinska J, Wasowicz W, Zbrog Z. Red blood cell and plasma glutathione peroxidase activities and selenium concentration in patients with chronic kidney disease: A review. Acta Biochim Pol. 2006;53:663–677.
- Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–519.
- Paul LC. Chronic renal transplant loss. Kidney Int. 1995;47:1491–1499.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Simic-Ogrizovic S, Simic T, Reljic Z, . Markers of oxidative stress after renal transplantation. Transpl Int. 1998;11(Suppl 1): 125–129.