Abstract
Induction therapy with interleukin-2 receptor antagonist (IL2RA) is widely used for renal transplant recipients and this study aimed to examine the impact of IL2RA among Chinese renal transplant recipients. Two hundred and thirty-eight Chinese renal transplant recipients aged 18–65 years at the Taichung Veterans General Hospital from January 2004 to July 2009 were retrospectively studied to assess the influence of IL2RA on biopsy-proven acute rejection (BPAR) within 1 year. Secondary outcomes included acute rejection rate in the first 3 months, delayed graft function, post-transplant diabetes mellitus, and malignancy. Cox proportional hazard analysis was used for multivariate analysis. Of all the patients, 116 received IL2RA (basiliximab, n = 44; daclizumab, n = 72) and 122 had no induction therapy. The mean follow-up duration was 43.3 months (range, 1–79 months). Overall, 227 (95.4%) patients completed the 12-month follow-up period with a functioning graft. No difference of BPAR was observed between the two groups and the secondary outcomes were also similar. After adjusting potential covariates with Cox regression, IL2RA use still provided no benefit on BPAR. In conclusion, there is no benefit of IL2RA in decreasing BPAR was observed in our study. Routine use of IL2RA for adult Chinese kidney transplant recipients may not be as effective as we thought before. More research is still needed to elucidate the effect of IL2RA among Chinese kidney transplant recipients.
INTRODUCTION
Monoclonal antibody targeting on the alpha subunit (CD25) of interleukin-2 receptor on activated T-lymphocytes includes basiliximab and daclizumab. The former is chimeric (human/mouse), while the latter is humanized antibody. They act as competitive antagonists with interleukin-2 and inhibit the proliferation and differentiation of T-lymphocytes.Citation1 Using two doses of basiliximab provides a 4–6 weeks blockade of the IL-2 receptor, while five doses of 1 mg/kg daclizumab preoperatively and post-transplant with 2 weeks interval provide adequate IL2 receptor saturation for more than 12 weeks.Citation2 Most of the acute rejection episodes occur in the early period after transplantation.Citation3 Therefore, it is reasonable to suggest that induction therapy with interleukin-2 receptor antagonist (IL2RA) in the perioperative period may decrease the early acute rejection rate and be helpful in long-term graft survival or patient survival. The impact of IL2RA on biopsy-proven acute rejection (BPAR) had been widely studied and reduced acute rejection rate in the short term had been observed in several researches.Citation4–7 Besides, some studies also revealed that the induction therapy may help lower the dosage of immunosuppressive agents.Citation8 However, there is paucity of such data on Chinese renal transplant recipients. This study aimed to determine the effect of IL2RA on adult Chinese kidney transplant recipients and to elucidate the impact of racial differences.
MATERIALS AND METHODS
Patients
All Chinese kidney transplant recipients aged 18–65 years who received kidney transplantation between January 2004 and July 2009, and those who were regularly followed up at Taichung Veterans General Hospital were included. The study was approved by the ethics committee of the Taichung Veterans General Hospital and was performed in accordance with the ethical standards. Patients were excluded if they received induction therapy with OKT3 (Muromonab), anti-thymocyte globulin (ATG), or anti-lymphocyte globulin (ALG); received multi-organ transplant; or ABO incompatible transplant. Patients with primary graft nonfunction were excluded. Those who received post-transplant ATG/ALG due to acute rejection were not excluded. All the deceased donors were standard criteria donors with brain death. No cardiac death donors or extended criteria donors were included in our study.
The study patients were categorized into the IL2RA and control groups. The former received induction therapy with basiliximab (Simulect®, Novartis, Basle, Switzerland) or daclizumab (Zenapax®, Hoffmann-La Roche, Basel, Switzerland) in the perioperative period, while the latter had no induction therapy. The use and selection of IL2RA was based on physician’s preference and patient’s ability to comply. Patient in the IL2RA group received 20 mg basiliximab on the operation day (day 0) and on day 4 post-transplantation or 1 mg/kg daclizumab on day 0 and day 14. The two-dose regimen of daclizumab was used instead of the five-dose regimen based on a previous preliminary analysis at our hospital, which showed comparable results of these two protocols (data not shown).
All patients received oral trimethoprim–sulfamethoxazole for Pneumocystis carinii prophylaxis for 6 months and oral valganciclovir for cytomegalovirus prophylaxis for 3 months.
Immunosuppressive Agents
All kidney transplant recipients received immunosuppressive agents with calcineurin inhibitor (CNI), mycophenolate mofetil (1.5 g/day), and prednisolone as initial regimen. The choice of using cyclosporine (CsA) or tacrolimus (TAC), with initial dose of 8 mg/kg/day and 0.2 mg/kg/day, respectively, was based on the clinical judgment of the attending physicians. Further adjustments were arranged according to the clinical settings. Drug level of TAC was kept around 8–12 ng/mL in the first year post-transplant, 5–8 ng/mL in the following years, and decreased to 3–5 ng/mL in the patients with stable long-term graft function. The whole blood trough level of CsA was kept around 200–300 ng/mL in the first 3 months post-transplant, 100–150 ng/mL in the following months, and decreased to 50–100 ng/mL 1 year after the operation.
All patients received preoperative and postoperative intravenous injection of 500 mg methylprednisolone. The dosage then was gradually tapered to 30 mg/day oral prednisolone on the day 4 post-operation, 15 mg/day by the end of first month, and to 5 mg/day by the end of the third month. Steroid use was kept to the minimum required dosage during subsequent outpatient visits. In some patients, sirolimus was added in the immunosuppressive regimen during follow-up, regardless of withdrawal of one of the other immunosuppressive agents.
Definitions
Delayed graft function (DGF) was defined as the indication for dialysis therapy within the first week after renal transplantation. All episodes of acute rejection were proven by renal biopsy, which was performed before or within 48 h after the initiation of antirejection treatment. Pathologic diagnosis was made according to the Banff criteria.Citation9,10 Borderline change followed by antirejection therapy was classified as acute rejection. Primary graft nonfunction was defined as failure of graft function recovery post-transplant. The estimated glomerular filtration rate (eGFR) was calculated via Cockcroft–Gault equation. Post-transplant diabetes mellitus (PTDM) was defined as newly developed hyperglycemia requiring treatment with oral antidiabetic drugs or insulin after renal transplantation. Post-transplant malignancy was defined as newly developed malignancy, which was diagnosed at least 3 months later after renal transplantation.
Outcomes
BPAR within the first year was the primary end point. The secondary end points were acute rejection rate in the first 3 months, incidence of DGF, time to acute rejection, serum creatinine, and eGFR at 1 year. PTDM and malignancy were also compared. We also compared the CNI dosage and blood drug level between the two groups in the 6th month and 1st year after the transplantation.
Statistical Analysis
All continuous data were expressed as mean ± SD and categorical data as number of patients (percent). The independent t-test was used to compare continuous data, while Fisher’s exact test or Yates’ correction for continuity was used for categorical data. Survival analysis with Kaplan–Meier curve and log-rank test were performed to evaluate the difference of 1-year acute rejection-free survival. Cox proportional hazards survival regression was used for multivariate analysis. Data were collected manually and inputted into an Excel spreadsheet (Microsoft, Seattle, WA, USA). All analyses were performed using the SPSS statistical software (version 15.0, SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered as statistically significant.
RESULTS
There were 269 kidney transplant patients during the study period. Among these, seven who received induction therapy with ATG, ALG, or OKT3, one who underwent ABO incompatible renal transplantation, and two with primary graft nonfunction due to graft kidney infarction were excluded. Another 21 were excluded due to age younger than 18 years or older than 65 years. Of the 238 adult Chinese kidney transplant recipients finally included, 116 received IL2RA and 122 had no induction therapy. In the IL2RA group, 44 received basiliximab and 72 received daclizumab.
Baseline demographic data were showed in . There was no difference in age, gender, pretransplant status, previous dialysis duration, viral hepatitis history, human leukocyte antigen (HLA) mismatch number, baseline CNI, and mammalian target of rapamycin inhibitor use. However, donors of the IL2RA group tended to be more living related, old, and female. Recipients of the IL2RA group had less percentage of retransplantation and high panel reactive antibody (PRA) (>20%). The mean follow-up duration after renal transplant was 43.3 months (range, 1–79 months). Overall, 227 (95.4%) patients completed the 12-month follow-up period with a functioning graft.
Table 1. Baseline demographic data.
The 1-year rejection-free survival was analyzed with Kaplan–Meier method and compared with log-rank test. There was no difference in the survival curve of the IL2RA and control groups (87.9% vs. 88.5%, p = 0.83) (). After dividing the IL2RA group into basiliximab and daclizumab subgroups (), there was still no difference compared with the control group (90.9% vs. 85.7% vs. 88.5%, p = 0.66). For patients with BPAR, the Banff classification was shown in . No significant difference in the severity was found when analyzed with Fisher’s exact test or Yates’ correction for continuity. One patient in the control group and two patients in the IL2RA group had graft loss even after aggressive medical treatment for acute rejection.
Figure 1. Kaplan–Meier curves for 1-year rejection-free survival between the interleukin-2 receptor antagonist (IL2RA) and control groups.
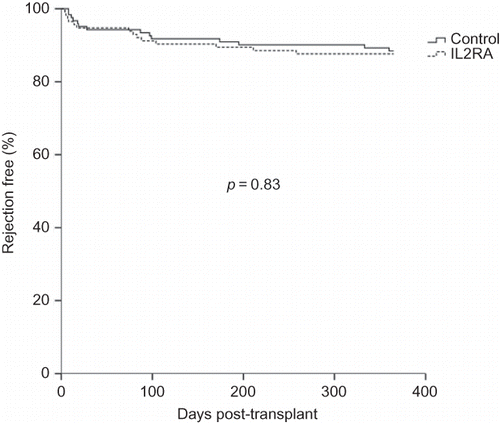
Figure 2. Kaplan–Meier curves for 1-year rejection-free survival among the basiliximab, daclizumab, and control groups.
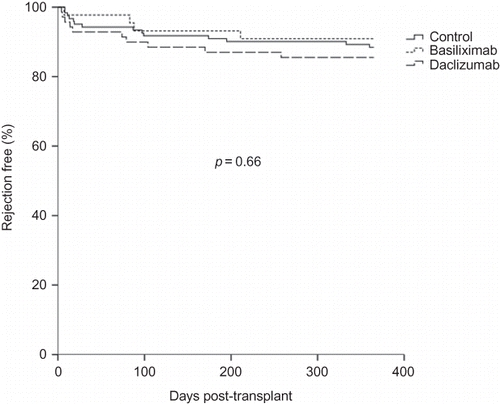
Table 2. Banff classification of BPAR at first year.
The secondary outcomes were summarized in . The acute rejection rate in the first 3 months was not different between the two groups. The IL2RA group had higher serum creatinine than the control group at 1 year (p = 0.008). However, there was no difference for eGFR calculated by the Cockcroft–Gault equation. DGF, time to acute rejection, occurrence of PTDM, occurrence of post-transplant malignancy, CNI dosage, and blood drug level were similar between the two groups.
Table 3. Secondary outcome measures.
To adjust for the effect of baseline demographic differences, Cox proportional hazard analysis was used for multivariate analysis, which showed no benefit on BPAR with regard to IL2RA even after the adjustments (). summarized the immunosuppressant conversion within the first year post-transplant and no obvious difference was observed.
Table 4. Cox proportional hazard for 1-year rejection-free survival.
Table 5. Summary of immunosuppressants conversion within the first year.
DISCUSSION
The baseline demographic data revealed that donors of the IL2RA group are more living related and older, with more female predominance than the control group. Recipients of the IL2RA group have less retransplantation and less high PRA, which is defined as PRA >20%. Most of the characteristics are favorable in the IL2RA group, which may overestimate the benefit of IL2RA in this study. However, there is still no benefit on acute rejection-free survival observed. Because IL2RA was known to be biologically active in the first 3 months after transplantation, we compared the acute rejection rate in the first 3 months and there is still no difference found.
In order to investigate further the effects of heterogeneity in the IL2RA group, it was further subdivided into the basiliximab and daclizumab groups. The Kaplan–Meier survival analysis still showed no differences between the three groups (p = 0.66). The measure of secondary outcome was comparable between the two groups and this confirmed the safety of IL2RA use in the clinical setting. Although there was higher serum creatinine in the IL2RA group, there was no difference in eGFR. In previous studies, IL2RA had been found to lower the dosage of immunosuppressive agents.Citation8 However, no significant differences in CNI dosage and blood level were observed in our data.
When used with CsA, IL2RA is associated with lower rate of acute rejection compared with placebo in previous studies.Citation1 There is no obvious difference in the first-year acute rejection rate between patients receiving basiliximab or daclizumab.Citation2,11 However, most of the studies revealed no benefit on long-term graft and patient survival with IL2RA.Citation5,6,12,13 Although the meta-analysis published in 2010 reveals less acute rejection rate in patients receiving IL2RA,Citation4 there is still some reports that have contradictory results.Citation14,15 TAC-based immunosuppressive regimens are suggested as the possible cause of differing results with previous studies on CsA-based immunosuppressive regimens.
In this study, most (80%) patients received TAC-based regimens. In order to clarify the influence of TAC, Cox regression was used and it showed no difference between TAC and CsA-based regimens as regards 1-year PBAR. Thus, advanced immunosuppressive regimens cannot completely explain the results of the study.
Ethnicity difference plays an important role in the immunologic risk profile of kidney transplant recipients. In previous studies, African-Americans have been associated with poor allograft survival compared with Caucasians.Citation16,17 In analysis of the United Network for Organ Sharing (UNOS) database, Asian recipients appear to have superior graft survival compared with Caucasians, Hispanics, and Blacks.Citation18 When renal transplant recipients are either Caucasians or Black, Asian donors also show better graft survival compared with Caucasian and Black donors. The impact of donor ethnicity on graft survival is not observable among Asian and Latino/Hispanic recipients.Citation19 The differences may be related to the lower immunologic risk among Asians. However, the influence of ethnicity difference may not limit to different immunological risk alone. A few studies also found ethnicity itself may be a risk factor for major cardiovascular event after renal transplantation.Citation20 The impacts of ethnicity disparity for renal transplantation is still an unresolved issue that worth further studies.
The efficacy of IL2RA in decreasing BPAR has been widely studied. Although the Cochrane meta-analysis published in 2010 shows positive effects of IL2RA on PBAR, only few studies have focused on the Chinese population.Citation4 Hence, the racial difference may not be revealed and further studies are warranted. The impact of IL2RA on Chinese kidney transplant recipients is less studied. Among 118 Chinese renal transplant recipients with CsA-based immunosuppressive regimen, Ji et al.Citation21 have found less BPAR (12.1% vs. 23.3%, p < 0.001) 6 months after transplantation in patients receiving single-dose daclizumab (n = 58) induction therapy. Zhu et al.Citation22 report using half-dose (0.5 mg/kg) daclizumab among 101 kidney transplant recipients in China and show decreased acute rejection within 6 months in those with good postoperative renal function recovery. The latter study does not mention the ethnicity of the recruited patients and provide no univariate and multivariate analysis data. This study provides a comprehensive analysis of IL2RA group with a control group in the largest number of Chinese patients reported to date.
This study has some limitations. First, two doses of 1 mg/kg daclizumab are used rather than the traditional five doses, which may lower its benefit. However, two doses of daclizumab are reportedly as efficacious as basiliximab.Citation11,23,24 Preliminary analyses in the study hospital also show no difference between the two- and five-dose regimens of daclizumab (data not shown). Therefore, the limited dose of daclizumab has been used to decrease treatment cost. Kaplan–Meier survival analysis also shows no difference between the basiliximab and daclizumab groups (). Second, this is a retrospective study. Hence, patient allocation is not randomized and baseline characteristics between groups may be unmatched. Besides, patients who were considered to have higher immunological risk may be more likely to be assigned to the IL2RA group in the clinical setting. However, Cox proportional hazard survival regression used to adjust for possible covariates still shows no benefit of IL2RA.
In conclusion, this retrospective study observed no benefit on BPAR of IL2RA among Chinese kidney transplant recipients under triple immunosuppressive agents. The results may be due to the lower immunologic risk among the Chinese population. Routine use of IL2RA for Chinese renal transplant recipients may not be as effective as we thought before. However, due to the limitation of retrospective study, more research is still needed to elucidate the effect of IL2RA among Chinese kidney transplant recipients.
ACKNOWLEDGMENT
The authors thank the biostatistics task force of Taichung Veterans General Hospital for their assistance in statistical analysis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Kahan BD, Rajagopalan PR, Hall M. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation. 1999;67:276–284.
- Kandus A, Arnol M, Omahen K, Basiliximab versus daclizumab combined with triple immunosuppression in deceased donor renal transplantation: A prospective, randomized study. Transplantation. 2010;89:1022–1027.
- Basadonna GP, Matas AJ, Gillingham KJ, Early versus late acute renal allograft rejection: Impact on chronic rejection. Transplantation. 1993;55:993–995.
- Webster AC, Ruster LP, McGee R, Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2010:CD003897.
- Ferrer F, Machado S, Alves R, Induction with basiliximab in renal transplantation. Transplant Proc. 2010;42:467–470.
- Sheashaa HA, Bakr MA, Rashad RH, Ismail AM, Sobh MA, Ghoneim MA. Ten-year follow-up of basiliximab induction therapy for live-donor kidney transplant: A prospective randomized controlled study. Exp Clin Transplant. 2011;9:247–251.
- Liu Y, Zhou P, Han M, Xue CB, Hu XP, Li C. Basiliximab or antithymocyte globulin for induction therapy in kidney transplantation: A meta-analysis. Transplant Proc. 2010;42:1667–1670.
- Ocampo C, Aristizabal A, Nieto J, Induction therapies in kidney transplantation: The experience of Hospital Pablo Tobon Uribe, Medellin, Colombia 2005–2010. Transplant Proc. 2011;43:3359–3363.
- Racusen LC, Solez K, Colvin RB, The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723.
- Solez K, Colvin RB, Racusen LC, Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–760.
- Aktas S, Colak T, Baskin E, Comparison of basiliximab and daclizumab with triple immunosuppression in renal transplantation. Transplant Proc. 2011;43:453–457.
- Sheashaa HA, Bakr MA, Ismail AM, Mahmoud KM, Sobh MA, Ghoneim MA. Basiliximab induction therapy for live donor kidney transplantation: A long-term follow-up of prospective randomized controlled study. Clin Exp Nephrol. 2008;12:376–381.
- Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: An analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90:1511–1515.
- Gavela Martinez E, Avila Bernabeu AI, Sancho Calabuig A, Beltran Catalan S, Escudero Quesada V, Pallardo Mateu LM. Use of basiliximab induction in low-immunological risk renal transplant recipients receiving tacrolimus-based immunosuppression. Transplant Proc. 2009;41:2337–2338.
- Cho WH, Lee HJ, Kim HT, Basiliximab does not reduce the early rejection incidence in high-risk kidney recipients under tacrolimus-based immunosuppression. Transplant Proc. 2008;40:2234–2236.
- Chakkera HA, O’Hare AM, Johansen KL, Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol. 2005;16:269–277.
- Zhou YC, Cecka JM, Terasaki PI. Effect of race on kidney transplants. Clin Transpl. 1990:447–459.
- Goh A. Graft survival trends in kidney transplants: An analysis of the UNOS database. Clin Transpl. 2009:41–54.
- Callender CO, Cherikh WS, Traverso P, Hernandez A, Oyetunji T, Chang D. Effect of donor ethnicity on kidney survival in different recipient pairs: An analysis of the OPTN/UNOS database. Transplant Proc. 2009;41:4125–4130.
- Prasad GV, Vangala SK, Silver SA, South Asian ethnicity as a risk factor for major adverse cardiovascular events after renal transplantation. Clin J Am Soc Nephrol. 2011;6:204–211.
- Ji SM, Li LS, Cheng Z, A single-dose daclizumab induction protocol in renal allograft recipients: A Chinese single center experience. Transplant Proc. 2007;39:1396–1401.
- Zhu YS, Xu AP, He HX, [Half-dose Zenapax for acute rejection prevention after renal transplantation]. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:1818–1820.
- Pham K, Kraft K, Thielke J, Limited-dose daclizumab versus basiliximab: A comparison of cost and efficacy in preventing acute rejection. Transplant Proc. 2005;37:899–902.
- Jain A, Valentini RP, Gruber SA, West MS, Mattoo TK, Imam AA. Two-dose daclizumab induction in pediatric renal transplantation. Pediatr Transplant. 2009;13:490–494.