Abstract
Background: The aim of the study was to confirm that glomerular hyperfiltration, an early and reversible stage of kidney damage, is associated in patients with prediabetes and prehypertension. Methods: In total, 5003 people aged between 35 and 69 years who had participated in the Shizuoka part of the Japan Multi-Institutional Collaborative Cohort (J-MICC) study took part in the study. Prevalence of hyperfiltration [the estimated glomerular filtration rate (eGFR) above the age- /sex-specific 95th percentile] was compared among different stages of prediabetes [fasting plasma glucose (FPG) < 100, 100–109, 110–125, and ≥126 mg/dL; and/or hemoglobin A1c (HbA1c) < 5.7, 5.7–6.0, 6.1–6.4 and ≥6.5% for no prediabetes, stage 1 prediabetes, stage 2 prediabetes, and overt diabetes, respectively] and prehypertension (blood pressure <120/80, 120–129/80–84, 130–139/85–89, and ≥140/90 mmHg for no prehypertension, stage 1 prehypertension, stage 2 prehypertension, and overt hypertension, respectively). Results: The prevalence of hyperfiltration increased with increasing stages of prediabetes (odds ratios: 1.25, 1.68, and 2.37 using FPG, and 1.26, 2.15, and 2.45 using HbA1c for stage 1 prediabetes, stage 2 prediabetes, and diabetes, respectively, relative to no prediabetes). Prehypertension, however, was not associated with hyperfiltration. Conclusion: The results confirmed that the prevalence of glomerular hyperfiltration increased with increasing stages (i.e., worsening) of prediabetes. Because both FPG and HbA1c showed similar association with hyperfiltration, either of these can be used to identify subjects who are at increased risk of nephropathy. Therefore, the functioning of kidneys should be monitored in subjects with prediabetes. Prompt treatment of hyperglycemia is necessary in subjects with hyperfiltration to prevent it to cause nephropathy.
INTRODUCTION
Prediabetes, defined as an impaired fasting glucose or impaired glucose tolerance,Citation1 and prehypertensionCitation2 have become widespread worldwide.Citation3 They are also considered to be the risk factors responsible for causing chronic kidney diseases.Citation4,5 Glomerular hyperfiltration, or an increased glomerular filtration rate (GFR), is a well-recognized early and reversible stage of kidney damage in subjects with diabetes and hypertension and is a marker for subsequent nephropathy progression.Citation6–10 Recently, it was reported that hyperfiltration is very prevalent in patients with prediabetes (and also in patients with prehypertension in our study) in several ethnic populations.Citation11–13 Identifying subjects who are at increased risk of nephropathy among those subjects with prediabetes and prehypertension offers an important and effective preventive strategy.
The American Diabetes Association recommends either fasting plasma glucose (FPG) or hemoglobin A1c (HbA1c) to evaluate prediabetesCitation1 because both predict the development of diabetes.Citation14 HbA1c scores over FPG as the subjects are not required to fast before its measurement. Therefore, it is important to compare how hyperfiltration and prediabetes associate using both FPG and HbA1c.
In view of this, the purpose of this study was to (1) confirm the association of hyperfiltration with prediabetes and prehypertension and (2) to compare the strength of associations according to the FPG and HbA1c levels.
SUBJECTS AND METHODS
Study Subjects
The subjects included in this study and their characteristics have been described elsewhere in more detail.Citation15,16 Briefly, the Japan Multi-Institutional Collaborative Cohort (J-MICC) study, a large cohort study, was established to examine gene–environment interactions in the context of lifestyle-related diseases. This study enrolled volunteers aged 35–69 years across 16 areas in Japan. The participants provided blood samples, and lifestyle data were recorded using questionnaires. The participants of this study were recruited in the Shizuoka part of the J-MICC study where they underwent health checkups at the Seirei Preventive Health Care Center (Hamamatsu City, Shizuoka Prefecture, Japan). All subjects between the age 35 and 69 years were invited to participate in the study between January 2006 and December 2007.
Subjects with complete data for the following characteristics were included in this study: serum creatinine (Scr), age, sex, FPG, HbA1c, and systolic/diastolic blood pressure (BP). These data, including height, weight, total and high-density lipoprotein (HDL) cholesterol, triglycerides, uric acid, and urinary protein levels, were obtained by undergoing health checkups. Blood samples were collected after α ≥8-h overnight fast. The HbA1c level values were converted from the Japan Diabetes Society values to the National Glycohemoglobin Standardization Program values. The subjects then completed self-administered questionnaires recording treatment for comorbid diseases, smoking status, and alcohol consumption. Subjects undergoing treatment for cancer were excluded from the study. Written informed consent was obtained from all subjects. The J-MICC study and its Shizuoka part were approved by the Ethics Committee of Nagoya University Graduate School of Medicine (approval numbers 253 and 288, respectively).
Estimation of the Glomerular Filtration Rate
SCr was measured in all participants using an enzymatic method. The GFR of each participant was estimated from the SCr value using the Modification of Diet in Renal Disease equation (adapted for Japanese individuals by the Japanese Society of NephrologyCitation17), as follows:
Definition of Hyperfiltration and Hypofiltration in Healthy Subjects
The distribution of estimated glomerular filtration rate (eGFR) in subjects without prediabetes (FPG < 100 mg/dL and HbA1c < 5.7%) or prehypertension (BP < 120/80 mmHg) was divided into 10-year age groups (or 35–39 years for subjects in their 30s). Subjects undergoing treatment for diabetes, hypertension, kidney diseases, and cancer, and those with proteinuria (urinary protein ≥ 1+ on dipstick test), were excluded from this analysis. Hyperfiltration was defined as an eGFR above the age- and sex-specific 95th percentile for apparently healthy subjects. Hypofiltration was defined as an eGFR below the 5th percentile.
Prevalence of Hyperfiltration and Hypofiltration according to the Stages of Prediabetes and Prehypertension
Using the resulting reference values, all participants were divided according to eGFR as showing hyperfiltration, normal filtration, and hypofiltration. The characteristics of the subjects were compared between subjects with hyperfiltration/hypofiltration and subjects with normal filtration. The prevalence of hyperfiltration and hypofiltration was also compared according to the stages of prediabetes and prehypertension.Citation1,2,18 The subjects were categorized into the following: normal fasting glucose (i.e., no prediabetes; FPG < 100 mg/dL), stage 1 prediabetes (FPG 100–109 mg/dL), stage 2 prediabetes (FPG 110–125 mg/dL), or diabetes (FPG ≥ 126 mg/dL or under treatment for diabetes).Citation1,16 Based on the HbA1c levels, the subjects were categorized into the following: no prediabetes (HbA1c < 5.7%), stage 1 prediabetes (HbA1c 5.7–6.0%), stage 2 prediabetes (HbA1c 6.1–6.4%), or diabetes (HbA1c ≥ 6.5% or under treatment for diabetes).Citation1,18 The subjects were also categorized into the following: normal BP (i.e., no prehypertension; BP < 120/80 mmHg), stage 1 prehypertension (BP 120–129/80–84 mmHg), stage 2 prehypertension (BP 130–139/85–89 mmHg), or hypertension (BP ≥ 140/90 mmHg or under treatment for hypertension).Citation2
Statistical Analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for hyperfiltration and hypofiltration using unconditional logistic regression analysis adjusted for age and sex (adjusted ORs), and for age, sex, body mass index, HDL–cholesterol, lipid-lowering medication use, uric acid, smoking status, and alcohol consumption. Because the stages of prediabetes and prehypertension are mutually confounding factors, the ORs were also adjusted for systolic BP and use of antihypertensive medications in the analyses of stages of prediabetes, and for FPG and use of glucose-lowering medications in the analyses of stages of prehypertension (fully adjusted ORs). The analyses were not adjusted for proteinuria because it is not just a confounder but is also an outcome, representing the severity of kidney damage. The p-values for trends were calculated using a categorical variable in which the absence of prediabetes, stage 1 prediabetes, stage 2 prediabetes, and diabetes or the absence of prehypertension, stage 1 prehypertension, stage 2 prehypertension, and hypertension was scored 0, 1, 2, and 3, respectively. p-Values < 0.05 were considered statistically significant. All analyses were carried out using STATA software version 9 (StataCorp, College Station, TX, USA).
RESULTS
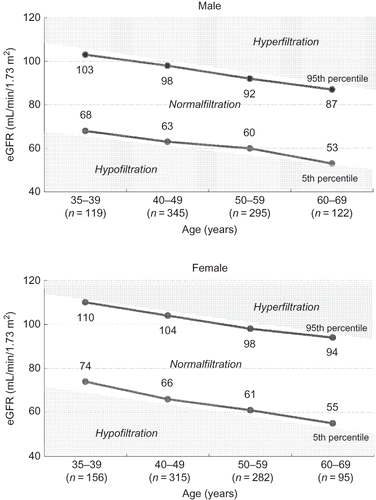
A total of 5040 people aged 35–69 years were enrolled into the study. Twenty subjects withdrew by the end of 2011. Seventeen subjects undergoing treatment for cancer were also excluded from the study. Finally, 5003 people (3407 males and 1596 females) were included in this study. The distribution of eGFR and the reference values for hyperfiltration/hypofiltration in subjects without prediabetes or prehypertension (n = 1729) for each 10-year age group are shown in . Among subjects aged 50–59 years, the reference values for hyperfiltration and hypofiltration were about 90 and 60 mL/min/1.73 m2 for males and 100 and 60 mL/min/1.73 m2 for females, respectively. The reference values were 1–7 mL/min/1.73 m2 lower in males than in females.
Figure 1. Distribution of eGFR in subjects without prediabetes or prehypertension by sex and age (n = 1729). The 95th and 5th percentiles are shown in 10-year age groups. Subjects with prediabetes (FPG ≥ 100 mg/dL), prehypertension (BP ≥ 120/80 mmHg), confirmed proteinuria (urinary protein ≥ 1+ on dipstick test), or who were being treated for diabetes, hypertension, renal diseases, or cancer were excluded from this analysis. Hyperfiltration was defined as an eGFR over the age- and sex-specific 95th percentile. Hypofiltration was defined as an eGFR below the 5th percentile.
The characteristics of subjects according to filtration status are shown in . Compared to subjects with normal filtration, subjects with hyperfiltration tended to have higher glycemic levels, lower lipid levels, and were more frequently current smokers and nondrinkers. In comparison, subjects with hypofiltration tended to have higher lipid and uric acid levels, more frequently used antihypertensive medications, and more frequently had proteinuria.
Table 1. Characteristics of subjects with hyperfiltration/hypofiltration compared with subjects with normal filtration (n = 5003).
Table 2. Prevalence of hyperfiltration/hypofiltration according to the stages of prediabetes and prehypertension in all subjects (n = 5003).
shows the prevalence of hyperfiltration and hypofiltration according to the stages of prediabetes and prehypertension. The prevalence of hyperfiltration increased with increasing stages of prediabetes defined by both FPG (fully adjusted ORs: 1.25, 1.66, and 2.40 for stage 1 prediabetes, stage 2 prediabetes, and diabetes, respectively; p for trend: <0.001) and HbA1c (fully adjusted ORs: 1.26, 2.12, and 2.45 for stage 1 prediabetes, stage 2 prediabetes, and diabetes, respectively; p for trend: <0.001). In contrast, no significant association was found between hyperfiltration and stages of prehypertension (ORs: 1.12, 1.12, and 1.25 for stage 1 prehypertension, stage 2 prehypertension, and hypertension, respectively; p for trend: 0.280). Hypofiltration was not associated with the stages of prediabetes or prehypertension.
DISCUSSION
In this study, it was confirmed that the prevalence of hyperfiltration increased with increasing stages of prediabetes in another large population. Notably, both FPG and HbA1c showed similar association with hyperfiltration.
Hyperfiltration, an early and reversible stage of kidney damage in subjects with diabetes, is a marker for subsequent nephropathy progression,Citation6–10 partly because of inappropriate afferent arteriole dilatation.Citation19 Recently, it was reported that hyperfiltration is prevalent among patients with prediabetes in various ethnic populations.Citation11–13 It is interesting to note that the association between hyperfiltration and prediabetes reported here was similar to that found in our previous study of about 100,000 Japanese individuals (ORs 1.29 and 1.58 for stage 1 prediabetes and stage 2 prediabetes, respectivelyCitation9). Moreover, hyperfiltration was more prevalent in subjects with prediabetes in two other studies, although the ethnic background and definition of hyperfiltration in those studies differed from our study. In a study of Caucasian individuals, hyperfiltration was defined as a GFR above the age-/sex-specific 90th percentile determined by iohexol clearance, and the resulting OR for prediabetes was 1.56.Citation12 In a study of African individuals, in which hyperfiltration was defined as GFR > 140 mL/min based on inulin clearance, the OR for prediabetes was 1.99.Citation13 To the best of our knowledge, these are the only studies to date to have reported an association between hyperfiltration and prediabetes. From these results, the association between hyperfiltration and prediabetes can be confirmed. Glomerular hyperfiltration might be a marker of early renal damage in prediabetes.Citation20
FPG and HbA1c both showed similar association with hyperfiltration. The American Diabetes Association recommends using either FPG or HbA1c as a diagnostic tool for prediabetes,Citation1 and subjects with an HbA1c of 5.7–6.4% should be informed of their increased risk for diabetes and cardiovascular disease.Citation21 A similar association was found between hyperfiltration and either FPG or HbA1c in an earlier study.Citation12 Our current results confirmed that either FPG or HbA1c can be used to identify subjects at increased risk of nephropathy.
There is no generally accepted definition for hyperfiltration.Citation22 We used age- and sex-specific reference values, as used in other studies,Citation11,12 because an uncorrected threshold for hyperfiltration would mask hyperfiltration in older subjects and in women.Citation12 Although the reference values of hyperfiltration differed from our previous study, the prevalence of hyperfiltration increased with increasing stages of prediabetes when previous reference values were used (ORs: 1.28, 1.38, and 2.65 for stage 1 prediabetes, stage 2 prediabetes, and diabetes, respectively; p for trend: <0.001). Thus, hyperfiltration should be defined using age- and sex-specific reference values of eGFR, and values that reflect the risk of nephropathy should be established in future investigations.
Interestingly, we could not confirm our previous study, which had showed an association between hyperfiltration and prehypertension.Citation11 The current study differs from our previous study in that the current subjects were all middle-aged. However, an association between hyperfiltration and prehypertension remained even when we re-analyzed the data from the previous study limited to subjects aged 35–69 years. Therefore, it is unclear whether the weak association between hyperfiltration and prehypertension in that study was real or was by chance, and this potential association should be evaluated in future studies. Consistent with our previous study, we found no association between hypofiltration and prediabetes/prehypertension in the current study. This suggests that prediabetes is associated with earlier stages of kidney damage (hyperfiltration) whereas long-term diabetes is associated with hypofiltration. Although hyperfiltration was associated with hyperglycemia, which is usually accompanied with hyperlipidemia, subjects with hyperfiltration showed lower lipid levels. This might be explained that subjects with hyperfiltration showed lower body mass index whose lipid levels were low.
Several limitations warrant mention. First, we estimated GFR rather than directly measuring GFR. Second, from these results, we could not determine whether treating hyperglycemia from the onset of hyperfiltration can prevent its progression to nephropathy, which should be examined in longitudinal studies. Third, age- and sex-specific eGFR reference values that reflect the risk of nephropathy should be established in prospective studies as the eGFR reference values for hyperfiltration and hypofiltration presented in this study need to be confirmed by eGFR change by prospective studies. Fourth, among subjects with normal filtration, subjects with renal damage who have already undergone hyperfiltration stage might be included in accordance with the hyperfiltration hypothesis.Citation8 As the hypofiltration group might be the result of late-stage kidney damage after hyperfiltration, the association of hyperfiltration will be greater if those subjects with hypofiltration were examined for GFR at an earlier period.
In conclusion, we have confirmed that the prevalence of hyperfiltration increases with increasing stage (i.e., worsening) of prediabetes. Either FPG or HbA1c can be used to identify subjects at increased risk of nephropathy. Therefore, the functioning of kidneys should be monitored in subjects with prediabetes, and prompt treatment of hyperglycemia is necessary in subjects with hyperfiltration to prevent it to cause nephropathy.
ACKNOWLEDGMENTS
The authors thank the following members of Seirei Preventive Health Care Center for their support in conducting this study: Hiromi Kaneko, Mika Tanaka, Tamiko Kozu, Akemi Ohnuma, Yoshie Yokoyama, Rie Suzuki, Tomomi Kawashima, Yasuko Nozue, Hisako Haruguchi, Ayako Mizuta, Motomi Ichikawa, Yukie Tsutsui, Erika Murakami, Misao Morishita, Hisae Fukatsu, Ayako Murasato, Yoko Yamashita, Midori Asai, Masami Taira, Miho Takai, Erina Koyama, Yoko Ohta, and Atsushi Koyama. This study was supported in part by a Grant-in-Aid for Scientific Research for Special Priority Areas of Cancer (No. 17015018) and Innovative Areas (No. 22150001) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69.
- Chobanian AV, Bakris GL, Black HR, ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572.
- Cowie CC, Rust KF, Ford ES, . Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294.
- Fox CS, Larson MG, Leip EP, Meigs JB, Wilson PW, Levy D. Glycemic status and development of kidney disease: The Framingham Heart Study. Diabetes Care. 2005;28:2436–2440.
- Munkhaugen J, Lydersen S, Widerøe TE, Hallan S. Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis. 2009;54:638–646.
- Mogensen CE. Glomerular hyperfiltration in human diabetes. Diabetes Care. 1994;17:770–775.
- Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691–697.
- Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777.
- Nelson RG, Bennett PH, Beck GJ, . Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335:1636–1642.
- Rudberg S, Persson B, Dahlquist G. Increased glomerular filtration rate as a predictor of diabetic nephropathy – An 8-year prospective study. Kidney Int. 1992;41:822–828.
- Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant. 2012;27:1821–1825.
- Melsom T, Mathisen UD, Ingebretsen OC, . Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care. 2011;34:1546–1551.
- Pruijm M, Wuerzner G, Maillard M, . Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol Dial Transplant. 2010;25:2225–2231.
- Sato KK, Hayashi T, Harita N, . Combined measurement of fasting plasma glucose and A1C is effective for the prediction of type 2 diabetes: The Kansai Healthcare Study. Diabetes Care. 2009;32:644–646.
- Hamajima N. J-MICC Study Group. The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev. 2007;8:317–323.
- Asai Y, Naito M, Suzuki M, . Baseline data of Shizuoka area in the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study). Nagoya J Med Sci. 2009;71:137–144.
- Matsuo S, Imai E, Horio M, . Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992.
- Ko SH, Kim SR, Kim DJ, .; Committee of Clinical Practice Guidelines, Korean Diabetes Association. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab. J. 2011;35:431–436.
- Sugimoto H, Shikata K, Matsuda M, . Increased expression of endothelial cell nitric oxide synthase (ecNOS) in afferent and glomerular endothelial cells is involved in glomerular hyperfiltration of diabetic nephropathy. Diabetologia. 1998;41:1426–1434.
- Palatini P. Glomerular hyperfiltration: A marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant. 2012;27:1708–1714.
- Ackermann RT, Cheng YJ, Williamson DF, Gregg EW. Identifying adults at high risk for diabetes and cardiovascular disease using hemoglobin A1c National Health and Nutrition Examination Survey 2005–2006. Am J Prev Med. 2011;40:11–17.
- Sunder-Plassmann G, Hörl WH. A critical appraisal for definition of hyperfiltration. Am J Kidney Dis. 2004;43:396.