Abstract
Objective: To elucidate the relationship of oxidative stress and specificity protein 1 (Sp1) in the process of epithelial-to-mesenchymal transdifferentiation (EMT) and also to investigate the molecular mechanism of protective effect of probucol on the pathogenesis of diabetic kidney disease (DKD). Methods: Thirty male Sprague–Dawley (SD) rats were randomly divided into control group, diabetic group, and diabetic group under probucol therapy (n = 10 per group). The biochemical indicators including 24-h urinary total protein (24-h UTP) excretion, blood glucose (BG), lipids [triglycerides (TGs), total cholesterol (TC)], serum creatinine (Scr), creatinine clearance rate (Ccr), kidney tissue malondialdehyde (MDA) level, and glutathione peroxidase (GSH-Px) activity were assessed in all groups. The renal pathological changes were evaluated by hematoxylin and eosin (HE) and Masson staining. The protein expression of Sp1, α-smooth muscle actin (α-SMA), and E-cadherin was also measured and analyzed by immunohistochemistry and Western blotting. Results: Compared with the control group, the BG, TC, Scr, 24-h UTP, and MDA level of renal tissue increased significantly and the Ccr reduced in the rats of diabetic group (all p < 0.01). The pathological scores and the expression of Sp1 and α-SMA in renal tissue were up-regulated (p < 0.01) and the expression of E-cadherin was down-regulated significantly in the diabetic animals (p < 0.01). In the diabetic animals treated with probucol, the renal injuries were alleviated (p < 0.01). Conclusions: Oxidative stress may play an important role in the EMT process of tubular epithelial cells. Probucol could ameliorate renal disease progression in this model of diabetic nephropathy, which might be due to an antioxidant action, down-regulation of Sp1 protein expression, and inhibition of renal tubular EMT.
INTRODUCTION
Diabetic kidney disease (DKD) is a microvascular complication of diabetes mellitus associated with significant morbidity and mortality.Citation1 It is characterized by the accumulation of extracellular matrix (ECM) in both the glomerular mesangium and the tubular interstitium, culminating in excessive renal scarring and a decline in excretory function.Citation2 Renal interstitial fibrosis is an inevitable pathologic event underlying progressive end-stage renal disease. The role of reversible epithelial-to-mesenchymal transdifferentiation (EMT) as a key regulator to renal fibrosis has been intensively investigated.Citation3,4 Recently, studies have showed that oxidative stress and nuclear transcription factor specificity protein 1 (Sp1) have been considerably linked to the pathogenesis of diabetic nephropathy.Citation5,6
Although strict control of hyperglycemia is possible with the use of several diabetic treatments, there are few effective agents that prevent the development of diabetic nephropathy.Citation7 Recently, several experimental studies indicated that probucol may have a beneficial effect in preventing the progression of early diabetic nephropathy.Citation8–10 Probucol is a cholesterol-lowering drug associated with reduced proteinuria and demonstrates anti-fibrotic, anti-inflammatory as well as strong antioxidant properties.Citation8,11 However, whether the protective effect of probucol on EMT of tubular epithelial cell mediated by oxidative stress is associated with the up-regulation of Sp1 in the pathogenesis of diabetic nephropathy is still uncertain. This study intended to elucidate the involvement of oxidative stress and Sp1 in the process of EMT associated with the pathogenesis of DKD, as well as the molecular mechanism underlying the putative renoprotective effect afforded by probucol.
MATERIALS AND METHODS
Reagents and Animals
The reagents used were rabbit anti-rat E-cadherin and Sp1 antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, USA), rabbit antibody against rat α-smooth muscle actin (α-SMA, Boaosen Biotechnology Co., Ltd., Beijing, China), malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), immunohistochemistry (IHC) anti-rabbit IgG secondary antibody assay kit (Shenzhen Jingmei Co., Ltd., Shenzhen, China), streptozotocin (STZ, Sigma, St. Louis, MO, USA), probucol (JingFuKang Co., Ltd., Beijing, China), bicinchoninic acid (BCA) assay kit (BiYunTian Co., Ltd., Jiangsu, China). Male Sprague–Dawley (SD) rats were purchased from laboratory animal center of Central South University.
Experimental Protocol
Thirty healthy 8-week-old male SD rats weighing 180–220 g were randomly separated into three groups: control group (C; n = 10), diabetic group (D; n = 10), and diabetic group under probucol therapy (P; n = 10), given food mixed at the concentrations of 1% probucol from the next day after the generation of the diabetes mellitus (DM) model. Diabetic rat model was induced by a single intraperitoneal injection of STZ (50 mg/kg body weight, freshly dissolved in 0.1 M citrate buffer), while control rats were injected with vehicle buffer only. The development of diabetes in two experimental groups was confirmed by measuring tail-vein blood glucose (BG) levels. Rats with non-fasting BG level of ≥20 mmol/L after 48 h of STZ injection were considered to be diabetes.Citation12 Six weeks after the STZ treatment has been shown to induce detectable diabetic complications in the kidney. During the duration of experiment, there were three dead rats: two in diabetic group and one in diabetic group under probucol treatment.
The 24 h urinary total protein (24-h UTP) excretion was collected and measured at the 3rd, 8th, and 12th week. At the end of the study, week 12, inferior caval vein blood samples were collected and renal tissue was dissected in all the rats under anesthesia, using an intraperitoneal injection of 10% chloral hydrate (3 mL/kg body weight). All experiments were approved by Medical Science Animal Care Committees of Central South University.
Blood and Urine Biochemical Data
At the end of treatments, week 12, BG, triglycerides (TGs), total cholesterol (TC), serum creatinine (Scr), and urine creatinine (Ucr) contents were assessed by Automatic Chemistry Analyzer of clinical laboratory in the Second Xiangya Hospital of Central South University. The creatinine clearance rate (Ccr) was calculated with standard formulas.
Renal Tissue MDA and GSH-Px Assay
MDA concentration and GSH-Px activity in the renal tissue were measured by using the commercial kit. In brief, 200-mg renal cortex was washed in ice-cold normal saline in tubes, cut into small pieces, and homogenized in ice-cold saline homogenization buffer at the proportion of 1:9 (v:w). The homogenate was centrifuged at 2000 rpm for 20 min at 4 °C. The supernatant was separated and analyzed for total protein and GSH-Px activity. The protein concentration of homogenate was determined using the BCA assay method. The results were expressed as nmol/mg protein (MDA) and U/g protein (GSH-Px), respectively. U = (2.3/(t2 – t1)) × log [GSH at t1 (1 min)/GSH at t2 (3 min)].
Pathological Evaluation
The right kidney was fixed in 10% buffered formalin and embedded in paraffin. The kidney samples were cut to sections of 4 μm and stained by hematoxylin and eosin (HE) and Masson staining. Glomerular and interstitial lesions were scored separately using a semiquantitative scoring system.Citation13 The relative collagen area of the tubulointerstitial region was observed and calculated by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Bethesda, MD, USA). The calculation was determined at 200× magnification in 50 fields for each animal. All histologic and morphometric determinations were performed double-blindly.
Renal IHC
IHC was performed on formalin-fixed, paraffin-embedded sections (4 μm). The slides were deparaffinized with xylene and graded concentrations of ethanol, and then rehydrated. After antigen retrieval and blocking, sections were incubated overnight at 4 °C with α-SMA (1:250), E-cadherin (1:200), and Sp1 (1:200) primary antibodies, respectively, followed by incubation with secondary antibody. Substrate [3,3-diaminobenzidine (DAB)] solution was prepared before use and added to the slides and then incubated in the dark for about 20 min. A negative control was included in which the primary antibody was preincubated with phosphate buffered saline (PBS) buffer. The expression of α-SMA, E-cadherin, and Sp1 protein in tubulointerstitium was quantified using a computerized image analysis system (Image-Pro Plus 6.0) by evaluating percentages of positive cell optical density relative to total field area in each field under the same light intensity as for microscopy. Each sample was performed triplicately and the average was taken as primary data of statistical analysis.
Western Blotting Analysis
The protein expression of α-SMA, E-cadherin, and Sp1 in renal tissue was detected by Western blotting. Briefly, each 100 mg sample of renal tissue was treated for 60 min with ice-cold lysis buffer and centrifuged at 10,000×g for 20 min at 4 °C; the supernatants were collected and stored at −70 °C. The protein concentration was determined by the BCA assay method. Subsequently, samples (50 μg of protein per lane) were loaded, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose (NC) filter membranes, and blocked by 1-h incubation at room temperature in TBS-T [tris-buffered saline (TBS) with 0.05% Tween 20, pH 7.4] with 5% BSA. Then, NC filter was incubated vibrating overnight at 4 °C with α-SMA and E-cadherin primary antibodies at dilution of 1:1000 in TBS-T with 5% milk. After thoroughly washed, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody at 1:1000 dilution in TBS-T with 5% milk. After multiple washes in TBS-T, specific proteins were detected using an enhanced electrochemiluminescence (ECL) detection system (Millipore Corporation, Billerica, MA, USA). Densitometric analysis of the membrane proteins was performed using IS4000MM chemiluminescence imaging system (Kodak, Rochester, NY, USA).
Statistical Analysis
Statistical analysis was performed using the SPSS software, version 10.0 (SPSS Inc., Chicago, IL, USA). The results were expressed as means ± standard deviation (![]() ). Data was evaluated using one-way analysis of variance (ANOVA) followed by a multiple comparison procedure (Fisher’s least significant difference test). A p-value of less than 0.05 was considered statistically significant.
). Data was evaluated using one-way analysis of variance (ANOVA) followed by a multiple comparison procedure (Fisher’s least significant difference test). A p-value of less than 0.05 was considered statistically significant.
RESULTS
Body Weight and UTP Excretion
Body weight and 24-h UTP excretion values were identical between the three groups at the baseline. In the diabetic rats, there was a trend of lower value of body weight, when compared with the control animals, after the 3rd week, which was significantly lower (p < 0.01) after the 8th week and at the final time (12 weeks). UTP excretion rate was significantly higher (p < 0.01) in the diabetic animals versus the control group throughout the study (weeks 3, 8, and 12)(). Probucol treatment has significantly (p < 0.01) prevented diabetes-induced body weight decrease and UTP excretion increment.
Table 1. Changes of body weight and 24-h UTP excretion in each group.
Biochemical Data and Oxidative Stress Parameters
As shown in , the diabetic rats presented, at the final time (12 weeks), hyperglycemia and significantly (p < 0.01) increased contents of TGs, TC, Scr, and kidney tissue MDA level, accompanied by reduced Ccr, when compared with the control animals. The diabetic rats under probucol treatment versus the diabetic animals showed significantly increased GSH-Px activity of kidney tissue (p < 0.01) and reduced Scr, TC, and MDA concentration (p < 0.01).
Table 2. Comparison of biochemical indicators and oxidative stress parameters at the end of 12th week in each group.
Renal Histomorphology
Glomerular lesions
Comparative analysis between normal control rats and diabetic animals at the end of 12th week revealed a significantly (p < 0.01) increased mesangial expansion, glomerulosclerosis, and glomerular atrophy in diabetic model animals. Glomerular injury was ameliorated after treatment with probucol (, ).
Figure 1. Comparison of renal histopathological changes at the end of 12th week in each group (HE × 100 and Masson × 200). C, control group; P, diabetic group under probucol therapy; D, diabetic group.
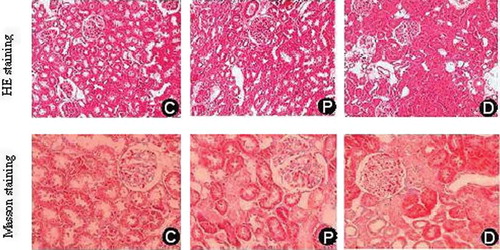
Table 3. Comparison of renal histopathological parameters at the end of 12th week in each group.
Tubulointerstitial and vascular lesions
The semiquantitative analysis showed that kidney/body weight (%), tubulointerstitial injury index, and the percentage areas of collagen staining in renal interstitium of diabetic rats were significantly higher versus the control animals or probucol-treated rats (p < 0.01), shown in and . Arteriolar hyalinosis was only found in the diabetic rats.
Kidney Protein Expression of α-SMA, E-cadherin, and Sp1
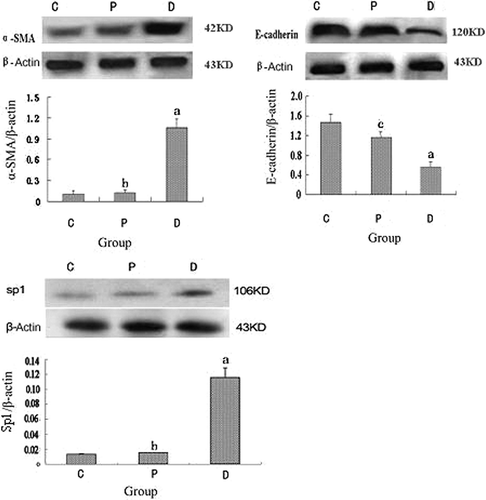
The IHC results indicated that the expression of α-SMA in normal control rats was merely detected in renal vessel wall, Sp1 in nucleus of tubular epithelial and mesangial cells, and positive E-cadherin signal in cell membrane and cytoplasm of renal tubule. As shown in , positive α-SMA and Sp1 signals of tubular epithelial cells detected in diabetic rats were significantly increased and the E-cadherin protein expression was decreased compared with those in control animals or probucol-treated rats (p < 0.01). However, the protein levels of α-SMA and Sp1 in renal tissue were down-regulated and the kidney E-cadherin expression was up-regulated in the animals with probucol treatment. Western blotting results, as shown in , demonstrated that the α-SMA and Sp1 protein expression in diabetic rats was significantly up-regulated and E-cadherin down-regulated compared with those in control animals (p < 0.01). The expression of renal tissue α-SMA and Sp1 protein was down-regulated and the kidney tissue E-cadherin expression was up-regulated in animals with probucol treatment (p < 0.01).
Figure 2. Protein expression of kidney tissue α-SMA, E-cadherin, and Sp1 in each group (immunohistochemistry × 200). C, control group;P, diabetic group under probucol therapy; D, diabetic group. ap < 0.01 versus control group; bp < 0.01 versus diabetic group.
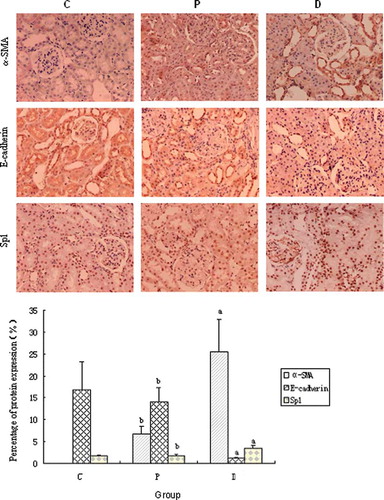
Figure 3. Protein expression of kidney tissue α-SMA, E-cadherin, and Sp1 in each group (Western blotting). C, control group; P, diabetic group under probucol therapy; D, diabetic group; KD, kilodalton. ap < 0.01 versus control group; bp < 0.01 versus diabetic group under probucol therapy; cp < 0.05 versus control group.
Correlation Analysis
The study showed that the MDA level of renal cortex was positively correlated with tubular injury score, percentage areas of interstitium collagen (γ = 0.803, p < 0.01; γ = 0.625, p < 0.01), and the protein expression of Sp1 and α-SMA in tubular epithelial cells (γ = 0.862, p < 0.01; γ = 0.896, p < 0.01, respectively), whereas the MDA level of renal cortex was negatively correlated with the E-cadherin expression (γ = −0.673, p < 0.01) in diabetic model rats. In addition, we found that the Sp1 protein expression is positively correlated with α-SMA in tubular epithelial cells (γ = 0.840, p < 0.01), but negatively correlated with the E-cadherin expression (γ = −0.636, p < 0.01) in diabetic model rats.
DISCUSSION
DKD is a leading cause of end-stage renal failure worldwide. EMT resulting in fibrosis with manifestation of tubular epithelial cells into myofibroblasts has been widely regarded as a key contributor to progressive diabetic nephropathy.Citation4,14 α-SMA-positive myofibroblasts from the tubular epithelial and mesenchymal phenotype transition mainly lead to the increased deposition of ECM in the pathology of tubulointerstitial fibrosis. Normally, tubular epithelial cells form a highly coupled epithelial sheet held together by E-cadherin. Loss of E-cadherin expression in the early stages of EMT results in the dissociation of cells within the epithelial sheet.Citation4
Oxidative stress has been known to play an important role in the development and progression of DKD.Citation15 Recent studies have suggested that oxidative stress is a key component in the development of dissociation of renal proximal tubular epithelial cell–cell junctions primarily through activation of mitogen-activated protein kinase (MAPK),Citation16 SmadCitation17,18, and/or extracellular signal-regulated kinase 1/2 (ERK1/2)Citation19 pathway in the early EMT. Sp1 is believed to play a critical role in the regulation of cell growth, differentiation, metastasis, and apoptosis.Citation6 It has been implicated to regulate glucose-mediated gene encoding profibrotic cytokine transforming growth factor-β (TGF-β) as well as genes in inflammation and ECM turnover. However, molecular mechanism regulating EMT of tubular epithelial cell mediated by oxidative stress is still uncertain. In recent studies, Sp1 is defined as an important fibrogenic factor involved in the accumulation of ECM during pathogenesis of diabetic nephropathy,Citation20,21 for instance, regulation of plasminogen activator inhibitor in STZ-induced diabetic rats. Although oxidative stress is known to play an important role in a series of EMT events, it is not known whether Sp1 is closely correlated with renal tubular EMT mediated by oxidative stress in diabetic nephropathy. Our study focused on the role of Sp1 in oxidative stress-mediated EMT. The results showed that in diabetic rat models we found decreased level of E-cadherin and increased level of Sp1 and α-SMA in renal tubular epithelial cell. Furthermore, we also demonstrated that there was positive correlation between kidney cortex levels of MDA and α-SMA, whereas there was negative correlation between MDA and E-cadherin. In addition, kidney protein expression of Sp1 is positively correlated with α-SMA and negatively with E-cadherin. These morphologic and phenotypic changes strongly suggested that EMT occurred in STZ-induced diabetic rats, further supporting oxidative stress, played a critical role in the early EMT of tubular epithelial cell. Our findings provided evidence for a role of Sp1 activated by oxidative stress in EMT and etiology investigation of diabetic nephropathy.
Recent studies showed that in STZ-induced diabetic rat model GSH-Px isoform is expressed in both normal and diabetic kidneys and protective against some types of injury caused by oxidative stress.Citation22,23 Undoubtedly, any treatment that is able to stabilize oxygen metabolism and modulate oxidative stress would help to delay the onset of DKD in diabetes mellitus. Probucol is a potent lipid-soluble antioxidant with two phenolic hydroxyl group that has been shown to reduce O2 production, scavenge lipid peroxidation, and inhibit DNA damage, although it is a cholesterol-lowering drug used clinically.Citation10 Recent studies have suggested that probucol may exert multiple favorable renal effect, including prevention of type-2 diabetes and disease progression in glomerulonephritis by antioxidant, inhibition of JAK2/STAT pathway, and antiproliferative effects in vivo and in vitro.Citation8,11,24–26 To date, there have been no reports about the morphological effect of probucol on the genesis of EMT and molecular mechanism underlying this effect. This study demonstrated that in STZ-induced diabetic rats the level of renal cortex MDA was positively correlated with the tubulointerstitial injury index and percentage areas of interstitium collagen. We also found a significantly reduction of MDA concentration, increased GSH-Px activity, down-regulated α-SMA and Sp1 protein expression, arrest of proteinuria, and amelioration of glomerular pathology in diabetes rats after treating with probucol. These observations suggested that probucol has a renoprotective effect on renal disease progression in this model of diabetic nephropathy. The mechanism might be involved in an antioxidant action, down-regulation of Sp1 protein expression, and inhibition of renal tubular EMT.
In conclusion, oxidative stress seems to play an important role in the EMT process of tubular epithelial cells. Probucol was able to ameliorate renal disease progression in this model of diabetic nephropathy, which might be due to an antioxidant action, as well as due to down-regulation of Sp1 protein expression and inhibition of renal tubular EMT.
ACKNOWLEDGEMENTS
The work was supported by grant from the Scientific Foundation of Hunan Province, China (2010FJ6008, 2008JT3005).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Soldatos G, Cooper ME. Diabetic nephropathy: Important pathophysiologic mechanisms. Diabetes Res Clin Pract. 2008;82(Suppl. 1):S75–79.
- Hills CE, Squires PE. The role of TGF-beta and epithelial-to-mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011;22:131–139.
- Hills CE, Al-Rasheed N, Al-Rasheed N, Willars GB, Brunskill NJ. C-peptide reverses TGF-beta1-induced changes in renal proximal tubular cells: Implications for treatment of diabetic nephropathy. Am J Physiol Renal Physiol. 2009;296:F614–F621.
- Hills CE, Squires PE. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol. 2010;31:68–74.
- Zhou Y, Liao Q, Luo Y, Qing Z, Zhang Q, He G. Renal protective effect of Rosa laevigata Michx by the inhibition of oxidative stress in streptozotocin-induced diabetic rats. Mol Med Report. 2012;5:1548–1554.
- Sanchez AP, Sharma K. Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev Mol Med. 2009; 11:e13.
- Kang MY, Park YH, Kim BS, Seo SY. Preventive effects of green tea (Camellia Sinensis var. Assamica) on diabetic nephropathy. Yonsei Med J. 2012;53:138–144.
- Du J, Wang L, Liu X, . Janus kinase 2/signal transducers and activators of transcription signal inhibition regulates protective effects of probucol on mesangial cells treated with high glucose. Biol Pharm Bull. 2010;33:768–772.
- Atsumi H, Kitada M, Kanasaki K, Koya D. Reversal of redox-dependent inhibition of diacylglycerol kinase by antioxidants in mesangial cells exposed to high glucose. Mol Med Report. 2011;4:923–927.
- Endo K, Miyashita Y, Sasaki H, . Probucol delays progression of diabetic nephropathy. Diabetes Res Clin Pract. 2006;71:156–163.
- Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003;14:S250–S253.
- Tesch GH, Nikolic-Paterson DJ. Recent insights into experimental mouse models of diabetic nephropathy. Nephron. 2006;104:e57–e62.
- Wang H, Li J, Yu L, Zhao Y, Ding W. Antifibrotic effect of the Chinese herbs, Astragalus mongholicus and Angelica sinensis, in a rat model of chronic puromycin aminonucleoside nephrosis. Life Sci. 2004;74:1645–1658.
- Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45.
- Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17: 4256–4269.
- Rhyu DY, Yang Y, Ha H, . Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16: 667–675.
- Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev (Orlando). 2008;22:1–5.
- Djamali A, Vidyasagar A, Adulla M, Hullett D, Reese S. Nox-2 is a modulator of fibrogenesis in kidney allografts. Am J Transplant. 2009;9:74–82.
- Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am J Physiol. 2007;293:F723–F731.
- Vayalil PK, Iles KE, Choi J, Yi AK, Postlethwait EM, Liu RM. Glutathione suppresses TGF-beta-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1281–L1292.
- Kang JH, Chae YM, Park KK, Kim CH, Lee IS, Chang YC. Suppression of mesangial cell proliferation and extracellular matrix production in streptozotocin-induced diabetic rats by Sp1 decoy oligodeoxynucleotide in vitro and in vivo. J Cell Biochem. 2008;103:663–674.
- De Haan JB, Crack PJ, Flentjar N, Iannello RC, Hertzog PJ, Kola I. An imbalance in antioxidant defense affects cellular function: The pathophysiological consequences of a reduction in antioxidant defense in the glutathione peroxidase-1 (gpx1) knockout mouse. Redox Rep. 2003;8: 69–79.
- Kornhauser C, Garcia-Ramirez JR, Wrobel K, Perez-Luque EL, Garay-Sevilla ME, Wrobel K. Serum selenium and glutathione peroxidase concentrations in type 2 diabetes mellitus patients. Prim Care Diabetes. 2008;2:81–85.
- Lee EA, Seo JY, Jiang Z, . Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 2005;67:1762–1771.
- Kondo S, Shimizu M, Urushihara M, . Addition of the antioxidant probucol to angiotensin II type I receptor antagonist arrests progressive mesangioproliferative glomerulonephritis in the rat. J Am Soc Nephrol 2006;17:783–794.
- Li G, Yin L, Liu T, . Role of probucol in preventing contrast-induced acute kidney injury after coronary interventional procedure. Am J Cardiol. 2009;103:512–514.