Abstract
Membranous nephropathy (MN), one of the most frequent causes of nephrotic syndrome in native kidneys, is also a common glomerular pathology in transplanted kidneys(Davison AM, Johnston PA. Allograft membranous nephropathy, Nephrol Dial Transplant, 1992;7(Suppl. 1):114–118. Specific treatment modalities have not been described for this population. However, renal transplanted patients presented with MN could have spontaneous remission as those with idiopathic MN. Here, we report a kidney allograft recipient diagnosed with de novo MN in early phases of posttransplantation period having a clinical remission over months.
INTRODUCTION
Membranous nephropathy (MN) is histologically characterized by thickening of the glomerular capillary due to immune-complex deposition in the glomerular basement membrane and clinically manifests with proteinuria and/or loss of renal function. It is one of the most frequent glomerular diseases seen in posttransplantation periodCitation1 and may lead to poor allograft survival.Citation2
In kidney allograft recipients, it may appear whether a new disease (de novo) or recurrence of initial renal disease, both of which have poorly understood heterogeneous pathogenetic mechanisms. Increased frequency of antibody-mediated rejection (AMR) episodes in patients with posttransplant de novo MN suggested that AMR might have a role in pathogenesis.Citation3 An association between hepatitis C virus infection and development of de novo MN in renal allografts was also manifested.Citation4
In different series, de novo MN has been reported to occur in 2–2.4% of the transplanted patients.Citation1,5 The exact prevalence has not been elucidated due to the differences in the indications of allograft biopsies among transplant centers and the sample sizes of the studies. The onset of proteinuria was reported at an average time of 19 months of transplantation.Citation6
The likelihood of spontaneous remission, a well-known characteristic of idiopathic MN, could also luckily be observed among patients with posttransplant MN.Citation7 Here, we report a case of de novo MN in relatively early phases of posttransplantation period having a clinical remission over months.
CASE REPORT
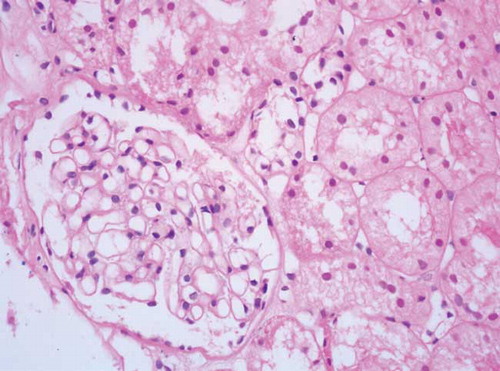
A 28-year-old male with hypertension-induced chronic renal failure (CRF) had a 3-year history of hemodialysis treatment and a 2-year history of peritoneal dialysis treatment. He received ABO-compatible deceased donor renal transplantation in September 2007. Daclizumab was used for induction and he was maintained on immunosuppressive therapy consisting of 5 mg/day of prednisolone, tacrolimus (twice daily; plasma level of 8–10 ng/mL), and 2 g/day of mycophenolate mofetil. Three months later, he presented with proteinuria of 900 mg/day. Complement and immunoglobulin levels were in normal ranges; anti-nuclear antibody, anti-ds-DNA, anti-glomerular basement membrane antibody, and anti-neutrophil cytoplasmic antibody were negative. Renal ultrasound revealed renal allograft with normal appearance. At fourth month of transplantation, proteinuria reached up to 2580 mg/day and allograft biopsy was performed. Some out of 16 glomeruli showed a thickening of glomerular basement membrane on light microscopy () and granular deposits of Ig G, lambda, and kappa light chains on immunofluorescence staining. The biopsy was reported as MN.
Figure 1. Light microscopic appearance of glomerulus from the patient with de novo MN (the capillary walls are diffusely thickened without an increase in mesengial cells or matrix; hematoksilen-eosin, original magnification ×400).
After the diagnosis, oral prednisolone dosage was increased to 0.5 mg/kg/day for 8 weeks and then tapered over months to a maintenance dosage of 10 mg/day. Additionally, irbesartan 300 mg/day, asetilsalisilic acid 100 mg/day, and atorvastatin 20 mg/day were prescribed. Serum creatinine level was stable, but proteinuria reached a peak value of 6800 mg/day in the following year. It started to decrease gradually after second year of transplantation. The patient is still in our follow-up with proteinuria of 372 mg/day and serum creatinine level of 1.0 mg/dL.
DISCUSSION
The presented case manifested with proteinuria at fourth month of transplantation with normal graft functions. Hypertension was thought to be the cause of kidney disease. However, it has been accepted as CRF without histological confirmation due to contracted kidneys. The absence of proteinuria and history of nephrotic syndrome symptoms before transplantation reduced the likelihood of MN as the cause of initial kidney disease. Hence, it was considered as de novo MN according to the allograft biopsy specimens.
It has been documented that patients with recurrent or de novo diseases are associated with prominently poor long-term survival.Citation2 Hence, this specific population warrants a particular attention, but there are no validated specific treatment modalities. Actually, in the setting of idiopathic MN, the convenient therapeutic approach and timing of immunosuppression were still controversial. In a retrospective study, it has been reported that steroid therapy did not decrease the level of proteinuria in de novo MN cases.Citation1 Schwarz et al.Citation8 showed that cyclosporine A regimen did not prevent the occurrence of de novo MN in allograft. Kossi et al.Citation7 reported spontaneous remission in a case of de novo GN with mycophenolate mofetil and an angiotensin receptor blocker. In recurrent MN cases, there are anecdotal case reports elucidating promising results after administration of rituximab.Citation9,10 Interestingly, Ideura et al. showed improved proteinuria after low-density lipoprotein apheresis in a patient with posttransplant MN.Citation11 For the presented case in this report, subsequent to the diagnosis of MN, steroid dosage was increased, and renin–angiotensin–aldosterone system blockers and antilipemic therapies were initiated. There is lack of data regarding the factors predicting clinical remission and poor outcome in this group of patients but reducing proteinuria with nonspecific therapeutic approaches could have favorable effects on the long-term prognosis. Spontaneous remission rate is rather low in posttransplant cases unlike idiopathic MN.Citation12 Our case experienced spontaneous remission after an average of 2 years.
We presented here a case of de novo MN relatively at earlier phases of posttransplantation period, who had clinical remission with preserved graft functions. This report may encourage clinicians that, although it is rare, spontaneous remission could also be achieved for patients with posttransplant MN like in those with idiopathic MN.12
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Schwarz A, Krause PH, Offermann G, Keller F. Impact of de novo membranous glomerulonephritis on the clinical course after kidney transplantation. Transplantation. 1994; 58(6):650–654.
- Hariharan S, Adams MB, Brennan DC, . Recurrent and de novo glomerular disease after renal transplantation: A report from Renal Allograft Disease Registry (RADR) Transplantation. 1999;68(5):635–641.
- Honda K, Horita S, Toki D, . De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transplant. 2011;25(2):191–200.
- Morales JM, Pascual-Capdevila J, Campistol JM, . Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation. 1997;63(11):1634–1639.
- Aline-Fardin A, Rifle G, Martin L, . Recurrent and de novo membranous glomerulopathy after kidney transplantation. Transplant Proc. 2009;41(2):669–671.
- Berger BE, Vincenti F, Biava C, Amend WJJr, Feduska N, Salvatierra OJr. De novo and recurrent membranous glomerulopathy following kidney transplantation. Transplantation. 1983;35(4):315–319.
- El Kossi M, Harmer A, Goodwin J, . De novo membranous nephropathy associated with donor-specific alloantibody. Clin Transplant. 2008;22(1):124–127.
- Schwarz A, Krause PH, Offermann G, Keller F. Recurrent and de novo renal disease after kidney transplantation with or without cyclosporine A. Am J Kidney Dis. 1991;17(5): 524–531.
- Gallon L, Chhabra D. Anti-CD20 monoclonal antibody (rituximab) for the treatment of recurrent idiopathic membranous nephropathy in a renal transplant patient. Am J Transplant. 2006;6(12):3017–3021.
- Weclawiak H, Ribes D, Guilbeau-Frugier C, . Relapse of membranous glomerulopathy after kidney transplantation: Sustained remittance induced by rituximab. Clin Nephrol. 2008;69(5):373–376.
- Ideura T, Hora K, Kaneko Y, et al. Effect of low-density lipoprotein-apheresis on nephrotic syndrome due to membranous nephropathy in renal allograft: A case report. Transplant Proc. 2000;32(1):223–226.
- Poduval RD, Josephson MA, Javaid B. Treatment of de novo and recurrent membranous nephropathy in renal transplant patients. Semin Nephrol. 2003;23(4):392–399.