Abstract
Chronic lymphocytic leukemia (CLL) is the most frequent form of leukemia in Western countries. Despite its relative frequency, the association of glomerular disease is extremely rare. We present a case of membranous nephropathy (MN) during CLL treated with fludarabine. A 74-year-old man was admitted to our hospital because of the onset of nephrotic syndrome (proteinuria was 7 g/24 h). Six years before, he had been diagnosed with CLL. Biochemical analysis showed the following results: creatinine was 1.7 mg/dL (creatinine clearance was 39 mL/min), urea was 64 mg/dL, hemoglobin was 8.6 g/dL, and white blood cells was 16,580/mm3 (60% lymphocytes). The urine sediment revealed 7–8 red blood cells and many hyaline and granular casts. No monoclonal peak was demonstrated in either serum or urine electrophoresis. Bence-Jones proteinuria was negative. The patient underwent renal biopsy that showed MN with an extensive lymphocyte perivascular infiltration; immunohistochemistry on renal biopsy specimen showed that infiltrating lymphocytes were CD20+. Moreover, DNA from tissue fractions was analyzed by qualitative polymerase chain reaction-based detection of clonal gene rearrangements of the immunoglobulin heavy chain gene, confirming the monoclonality of the infiltrating lymphocytes. The patient was started on fludarabine as monotherapy, with complete remission of proteinuria and recovery of renal function (creatinine clearance was 75 mL/min) after 1 year of follow-up.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most frequent form of leukemia in Western countries characterized by the clonal proliferation and accumulation of neoplastic B lymphocytes in the blood, bone marrow, lymph nodes, and spleen. The median age of patients at diagnosis is 65 years, and in most series, more men than women are affected. The course of the disease is variable. Whereas some patients with CLL have a normal life span, others die within 5 years after diagnosis.Citation1
Direct involvement of kidney parenchyma by lymphoplasmacytic neoplasms is relatively common, especially in the advanced stage of the disease. In some autopsy series, the prevalence of renal involvement was found in up to 90% of patients.Citation2 Despite the relative frequency of this type of leukemia, the association of glomerular disease is extremely rare. In 1957, Scott first reported the association of the nephrotic syndrome (NS) with CLL. Since then, there have been occasional case reports of patients with CLL and the NSCitation3 that still remains a rare complication, occurring in less than 2% of patients with CLL. The most common type (35.7%) of glomerulonephritis causing NS during CLL is Type I and II membranoproliferative glomerulonephritis (MPGN), while less frequent causes include membranous nephropathy (MN), minimal-change disease, focal segmental glomerulosclerosis, focal or diffuse proliferative glomerulopathy, amyloidosis, and immunotactoid glomerulonephritis.Citation4 To date, only 12 cases of MN complicating CLL have been reported in literature.Citation2,4–13 We present another case of MN during CLL treated with fludarabine.
CASE REPORT
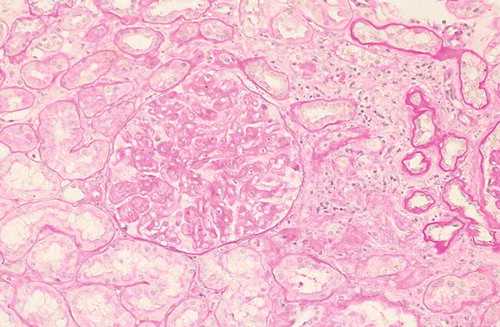
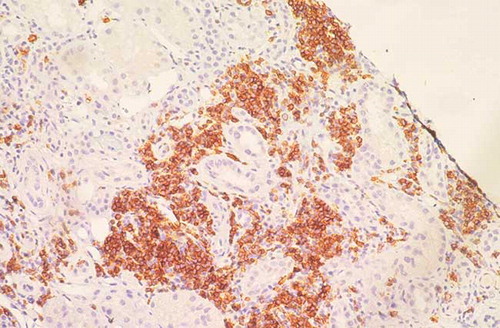
A 74-year-old man was admitted to our hospital in April 2011 because of edema of his lower extremities, with weight gain of 10 kg during 1 month. Six years before, he had been diagnosed with CLL. Bone marrow aspiration and biopsy were consistent with a diagnosis of CLL with a dense, mature lymphocytic infiltrate (60%) throughout the bone marrow. Flow cytometry immunophenotyping demonstrated a clonal B cell population CD38+, ZAP 70–, with surface λ chain expression consistent with B-CLL. At that time, no therapy was started. Physical examination upon admission showed no significant lymphadenopathy or hepatosplenomegaly and his blood pressure was 120/80 mmHg. Chest X-rays and abdominal ultrasounds were normal. Biochemical analysis showed the following results: creatinine was 1.7 mg/dL (creatinine clearance was 39 mL/min), urea was 64 mg/dL, total protein was 4.8 g/dL, serum albumin was 2.4 g/dL, hemoglobin was 8.6 g/dL, white blood cells was 16,580/mm3 (60% lymphocytes), and platelets was 179,000/mm3. Blood glucose, hepatic tests, lipids, and coagulation were normal. The urine sediment revealed 7–8 red blood cells and many hyaline and granular casts. Urinary protein excretion was 7 g/24 h. No monoclonal peak was demonstrated in either serum or urine electrophoresis. Bence-Jones proteinuria was negative. The third (C3) and fourth (C4) components of complement were normal. Antinuclear antibody, extractable nuclear antigens, perinuclear Anti-Neutrophil Cytoplasmic Antibody (pANCA), and cytoplasmic Anti-Neutrophil Cytoplasmic Antibody (cANCA) tested negative. Cryoglobulins were not detected in serum. Hepatitis B, hepatitis C, and HIV serology were also negative. The direct and indirect Coombs’ tests were negative. A percutaneous renal biopsy was performed. Twenty glomeruli were evaluated; focal segmental sclerosis was detected in two glomeruli. The remaining showed diffuse thickening of glomerular basement membrane (). Furthermore, extensive lymphocyte perivascular infiltration was present; immunohistochemistry on renal biopsy specimen showed that infiltrating lymphocytes were CD20+ (). Immunofluorescent staining revealed deposition of IgG, IgM, C3, C1q, and λ chains. DNA from renal tissue fractions with infiltrating cells was analyzed by qualitative polymerase chain reaction (PCR)-based detection of clonal gene rearrangements of the immunoglobulin heavy chain gene. This test provided direct evidence of monoclonality of infiltrating lymphocytes. Final diagnosis was “membranous nephropathy associated with renal localization of CLL.” The patient was started on fludarabine as monotherapy; 2 months later, proteinuria decreased to 4 g/24 h and 1 month later, complete remission was achieved. After 1 year of follow-up, remission of proteinuria was stable and renal function was recovered (creatinine clearance was 75 mL/min).
DISCUSSION
Renal impairment with or without proteinuria may be the first manifestation of clinically silent systemic lymphoplasmacytic neoplasms. Obstruction of the ureters, sepsis, hemolysis, and therapy-related toxicity are the most common causes of renal disease in patients with these diseases.Citation4 Glomerulonephritis with NS rarely occurs in patients with CLL and a variety of glomerular lesions have been observed. While the majority of patients with CLL-associated NS have been found to have MPGN, MN, a common cause of NS in adults and the most common glomerulonephritis in carcinomas, is rarely associated with CLL, despite the relative frequency of both diseases. To the best of our knowledge, only 12 cases of MN during CLL have been reported in literature (summarized in ). Overall, the data demonstrate that the occurrence of GN in CLL is not fortuitous. It rather testifies to the paraneoplastic nature of glomerular involvement. Broad generalizations regarding the pathogenesis of renal lesions in these diseases are difficult, partly due to the paucity and sporadic reporting of such cases. Mechanisms proposed to explain the renal pathologic findings include, but are not limited to, autoimmunity due to T-cell dysregulation, hyperglobulinemia, cytokine-induced altered glomerular permeability, Vascular Endothelial Growth Factor (VEGF) and Transforming Growth Factor (TGF) β 1-induced glomerulosclerosis, dysproteinemia with or without cryoglobulinemia, followed by immune complex formation and subsequent immune complex deposition in glomeruli, viral infections, toxins, and others.Citation2 The demonstration of immunoglobulins and complement in paraneoplastic glomerular lesions, even in the absence of monoclonal component and complement consumption, suggests that the deregulation of humoral and cellular immunity that characterizes CLL contributes to MN. The production of autoantibodies that recognize renal epithelial cell structure as well as altered glomerular permeability caused by cytokines produced by T-cells are postulated to participate in the genesis of MN.Citation4 Interstitial lymphocytic infiltration occurs in 50–60% of patients with CLL. Recognition of neoplastic nature of small infiltrates may be at times challenging. The monotonous appearance of the infiltrating cell population, their uniform size, and shape, as well as their destructive nature should prompt the examiner to perform additional workup to rule out or confirm the diagnosis. A series of immunohistochemical stains should be performed to establish (a) the lineage of cell type, (b) clonality, and (c) specific gene rearrangement.Citation2 In our patient, extensive lymphocyte perivascular infiltration was present; immunohistochemistry on renal biopsy specimen showed that infiltrating lymphocytes were CD20+. Moreover, DNA from tissue fractions was analyzed by qualitative PCR-based detection of clonal gene rearrangements of the immunoglobulin heavy chain gene, confirming the monoclonality of the infiltrating lymphocytes. Fludarabine is probably the single most active agent in CLL and is now recommended as first-line therapy for this disease. F-ara-A, the active compound of fludarabine, has a complex mechanism of action.Citation11 In resting lymphocytes, the accumulated triphosphate fludarabine derivatives result in defects in the repair of DNA, DNA strand breaks, activation of endogenous nucleases, depletion of nicotinamide adenine dinucleotide , and apoptotic cell death. In dividing lymphocytes, these agents inhibit the ribonucleotide reductase that ultimately leads to the inhibition of DNA synthesis.Citation13 Our patient was treated with fludarabine, with complete remission of proteinuria and recovery of renal function. So far, fludarabine was used in this case and in two previous cases, showing that this drug may be considered as a reasonable and efficacious alternative to traditional alkylator-based therapy in patients with severe membranous glomerulopathy coexistent with CLL.Citation13 Remission induction of glomerular disease with successful treatment of the leukemia provides further indirect evidence of the paraneoplastic nature of the glomerular involvement.
Table 1. Cases of MN in patients with CLL.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052–1057.
- Kowalewska J, Nicosia RF, Smith KD, Kats A, Alpers CE. Patterns of glomerular injury in kidneys infiltrated by lymphoplasmacytic neoplasms. Hum Pathol. 2011;42(6):896–903.
- Seney FD Jr, Federgreen WR, Stein H, Kashgarian M. A review of nephrotic syndrome associated with chronic lymphocytic leukemia. Arch Intern Med. 1986;146(1):137–141.
- Ziakas PD, Giannouli S, Psimenou E, Nakopoulou L, Voulgarelis M. Membranous glomerulonephritis in chronic lymphocytic leukemia. Am J Hematol. 2004;76(3):271–274.
- Cameron S, Ogg CS. Nephrotic syndrome in chronic lymphatic leukaemia. Br Med J. 1974;4:164.
- Row PG, Cameron JS, Turner DR, . Membranous nephropathy. Long-term follow up and association with neoplasia. Q J Med. 1975;44:207–239.
- White CA, Dillman RO, Royston I. Membranous nephropathy associated with an unusual phenotype of chronic lymphocytic leukemia. Cancer. 1983;52:2253–2255.
- Touchard G, Preud’homme JL, Aucouturier P, . Nephrotic syndrome associated with chronic lymphocytic leukemia: an immunological and pathological study. Clin Nephrol. 1989;31:107–116.
- Moulin B, Ronco PM, Mougenot B, Francois A, Fillastre JP, Mignon F. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992;42(1):127–135.
- Eisterer W, Neyer U, Hilbe W, . Effect of cyclosporin A in a patient with refractory nephrotic syndrome associated with B chronic lymphocytic leukemia. Nephron. 1996;72:468–471.
- Butty H, Asfoura J, Cortese F, Doyle M, Rutecki G. Chronic lymphocytic leukemia-associated membranous glomerulopathy: remission with fludarabine. Am J Kidney Dis. 1999;33:E8.
- Da’as N, Polliack A, Cohen Y, . Kidney involvement and renal manifestations in non-Hodgkin’s lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Hematol. 2001;67:158–164.
- Abdel-Raheem MM, Jamil MG, Potti A, Gross GG. Fludarabine therapy in chronic lymphocytic leukemia-associated severe nephrotic syndrome. Hematologica. 2000; 85(11):1224–1225.