Abstract
In this article, we present the case of a man with uremia. Laboratory testing revealed thrombocytopenia, erythrocyte fragmentation, elevated lactate dehydrogenase, and malignant hypertension, manifestations that are similar to thrombotic microangiopathy (TMA). Thromboasthenia, manifested as a decrease in the platelet aggregation rate, was also noted. Regular hemodialysis (3 times per week) improved the patient’s thrombocytopenia and thromboasthenia. This case supports the conclusion that uremic toxin, which can be removed by hemodialysis, inhibits the quantity and quality of platelets. We believe that the platelet aggregation rate can be a useful tool in distinguishing uremia from TMA.
INTRODUCTION
The pathophysiology and treatment of uremia and thrombotic microangiopathy (TMA) are significantly different; however, they both involve the kidneys and may manifest as thrombocytopenia, erythrocyte fragmentation, and malignant hypertension. We sought a useful tool for the differential diagnosis of these two disorders. In this article, we present the case of a man with uremia and features of TMA. The ultimate diagnosis was obtained by the examination of the patient’s platelet aggregation rate.
CASE REPORT
A 24-year-old male complained of nausea, weakness, headache, and dizziness lasting longer than 3 weeks. Two weeks ago, the patient visited the Department of Digestion but could not be diagnosed. One day later, he visited the Department of Ophthalmology complaining of visual disturbances and was diagnosed with hypertensive retinopathy.
On his visit to the Department of Ophthalmology, the patient’s blood pressure was 220/130 mmHg (equal in both arms) with a respiration rate of 20/min, the heart rate was 72 beats/min, and the temperature was 36.1°C. A physical examination produced normal results without focal neurological deficits or edema of either lower extremity. An ophthalmologic examination showed intraretinal hemorrhaging in both eyes, compatible with grade III hypertensive retinopathy (). The laboratory test results revealed normocytic anemia, thrombocytopenia, thromboasthenia, and renal failure (). Thromboasthenia was manifested as hemorrhinia and a decline in the adenosine-diphosphate- induced platelet aggregation rate. However, coagulant function testing, including prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen, and international normalized ratio, was normal. Blood coagulation factors III, V, VII, VIII, IX, and X were normal. The patient’s lactate dehydrogenase (LDH) level (269 U/L, normal reference level = 115–245 U/L) was high; however, his total bilirubin concentration (6.0 μmol/L, normal reference level 2–19 = μmol/L) was normal. Urinalysis of a spot urine sample produced the following results: albumin was 1+; red blood cell count was >30/high power field; and white blood cell count was 1–3/high power field. A peripheral blood smear showed 2% schistocytes. The patient tested negative for human immunodeficiency virus, viral hepatitis, and sickle cell disease. He was tested negative for antinuclear antibody and ds-DNA and a bone marrow examination produced normal results. Electrocardiograms and chest X-rays were unremarkable. A computed tomography scan of the head was negative for intracranial hemorrhage.
Table 1. The platelet aggregation rate was tested at different time points during treatment.
Figure 1. Intraretinal hemorrhage noted before treatment compatible with grade III hypertensive retinopathy.
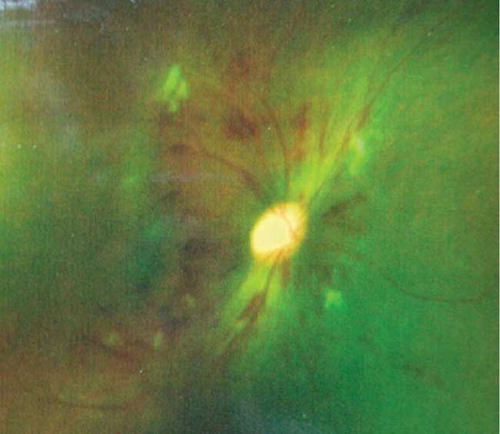
Figure 2. Renal biopsy showing tubulointerstitial nephropathy. (A) Glomerulus with a focal necrotizing lesion and fibrous crescent (periodic acid Schiff-hematoxylin stain; ×100 magnification). (B) Tubulointerstitial lesion manifested as diffuse interstitial fibrosis and tubular atrophy (Masson stain; ×100 magnification).
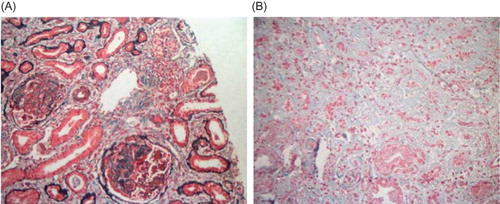
The patient was transferred to the Department of Nephrology because of renal failure. There, the patient was administered nifedipine, terazosin, metoprolol, valsartan, and furosemide to control his blood pressure. The patient’s systolic blood pressure ranged from 150 to 120 mmHg and his diastolic blood pressure ranged from 90 to 80 mmHg, despite an aggressive antihypertensive regimen. After his blood pressure was stabilized, the patient’s thrombocytopenia and thromboasthenia failed to improve. A renal biopsy showed tubulointerstitial nephropathy. The lesions were manifested by glomerulosclerosis, a fibrous crescent, diffuse interstitial fibrosis, and tubular atrophy. Alterations in the renal vasculature included arteriosclerosis, intimal and medial hypertrophy, and hyalinization. The renal biopsy did not show any evidence of renal TMA (). Over 40 days of regular hemodialysis 3 times a week, the patient’s hemoglobin level, platelet count, and platelet aggregation rate returned to normal.
DISCUSSION
This patient manifested thrombocytopenia, erythrocyte fragmentation, elevated LDH, and malignant hypertension; these were the features of TMA. TMA is defined as a lesion that causes partial or complete obstruction of the vessel lumina via microvascular platelet aggregation. Intrarenal TMA is a typical histological finding in malignant hypertension with renal impairment.Citation1 TMA may manifest as hemolytic uremic syndrome (HUS), thrombotic thrombocytopenic purpura (TTP), or malignant hypertension.
TTP is a disorder characterized by TMA, thrombocytopenia, neurologic abnormalities, acute renal insufficiency, and fever. HUS refers to a form of TMA that affects young children with an Escherichia coli infection and involves the kidneys. Patients with TTP/HUS typically have severe thrombocytopenia (platelet count <20,000/μL),Citation2 but not severe hypertension.
Malignant hypertension is a clinical syndrome characterized by severe hypertension, multiorgan dysfunction, and activation of the renin–angiotensin–aldosterone system and which is often associated with microangiopathic hemolytic anemia. Organ dysfunction, including encephalopathy, renal dysfunction, myocardial ischemia, retinal hemorrhage, exudates, or papilledema, is a common finding in malignant hypertension. The pathology of organ damage in malignant hypertension is typically characterized by endothelium damage, fibrinoid necrosis, fibrin, and platelet clots in the lumina of arterioles, leading to erythrocyte fragmentation and platelet consumption.Citation3 In malignant hypertension patients, high blood pressure may be the cause of the fragmentation of erythrocytes. High blood pressure persists for a longer duration and can result in microvascular injury, endothelial damage, and lumen narrowing. When passing through the narrowed vascular lumen under high shear stress, erythrocytes can fragment. Following this event, LDH is elevated.
The renal biopsy of the patient did not show evidence of renal TMA, but tubulointerstitial nephropathy. After physical and laboratory examinations, he was diagnosed with uremia. Meanwhile, he has the same manifestations as the TMA. The question, therefore, is whether he had TMA. In this situation, it was necessary to distinguish TMA from uremia. We found that the platelet aggregation rate is an effective method. The pathophysiology of the abnormal bleeding in uremia is thought to be multifactorial.Citation4 The coagulation protein cascade and platelets are normal,Citation5,6 although a prolonged bleeding time and abnormalities in platelet function are features of uremia. The platelet aggregation rate is an indicator of bleeding and platelet adhesion. In the initial phase of platelet aggregation, the binding of von Willebrand factor (VWF) to platelet membrane glycoprotein (GP) Ib under high shear stress causes the opening of transmembrane Ca2+ channels, resulting in an inward Ca2+ flux in platelets. The increased intracellular Ca2+ level activates processes that lead to a functional change in GPs IIb and IIIa, allowing them to bind to VWF. The binding of VWF to GPs IIb and IIIa mediates platelet aggregation. Urea has been reported to inhibit platelet aggregationCitation7; however, the opposite result has also been reported.Citation8 In our patient, the platelet aggregation rate improved, although his urea level did not change significantly. It seems therefore possible that the decrease in platelet aggregation was due to the accumulation of substances other than urea. The fact that the patient’s platelet abnormalities could be corrected by hemodialysis supports the notion that these substances are small molecular toxins that may act on peripheral platelets rather than bone marrow, because the patient’s bone marrow was normal on examination. Nakamura et al.Citation9 reported that uremic platelet dysfunction results from decreased availability of GPs IIb and IIIa due to fibrinogen and VWF fragments. In uremic plasma, the clearance of fibrinogen and VWF fragments is decreased and the activity is enhanced. These fragments can act as “putative uremic toxins” that inhibit VWF binding to GPs IIb and IIIa, which may be the main reason for the decrease in the platelet aggregation rate.Citation10 Hemodialysis can improve the platelet aggregation rate by removing fibrinogen and VWF fragments. The quantity of platelets in our patient returned to normal after regular hemodialysis; therefore, we speculate that these uremic toxins can destroy peripheral platelets.
The pathophysiology of the thrombocytopenia in TMA patient is due to the formation of microthrombus leading to platelet consumption. However, in uremic patients, uremia toxins can lead to thrombocytopenia and platelet dysfunction. Platelet aggregation rate is a common tool to detect the function of platelets. An abnormal platelet aggregation rate is relatively common in uremia, but it is uncommon in TMA. Therefore, the platelet aggregation rate can be a useful tool for distinguishing these conditions.
CONCLUSIONS
It is rather hard to distinguish uremia from TMA in clinic because of similar manifestations, including thrombocytopenia, erythrocyte fragmentation, elevated LDH, and malignant hypertension. However, through analyzing the different pathophysiologies of uremia and TMA, we have found that the platelet aggregation rate is a more practical tool for distinguishing between the two kinds of diagnoses to supply appropriate treatment compared with renal biopsy, which is an invasive method.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411–417.
- Elliott MA, Nichols WL. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Mayo Clin Proc. 2001;76(11):1154–1162.
- Khanna A, McCullough PA. Malignant hypertension presenting as hemolysis, thrombocytopenia, and renal failure. Rev Cardiovasc Med. 2003;4(4):255–259.
- Rabelink TJ, Zwaginga JJ, Koomans HA, Sixma JJ. Thrombosis and hemostasis in renal disease. Kidney Int. 1994;46(2):287–296.
- Larsson SO. On coagulation and fibrinolysis in uremic patients on maintenance hemodialysis. Acta Med Scand. 1971; 189(6):453–462.
- Gafter U, Bessler H, Malachi T, Zevin D, Djaldetti M, Levi J. Platelet count and thrombopoietic activity in patients with chronic renal failure. Nephron. 1987;45(3):207–210.
- Hellem A, Owren PA. The mechanism of the hemostatic function of blood platelets. Acta Hematol. 1964;31:230–238.
- Castaldi PA, Rozenberg MC, Stewart JH. The bleeding disorder of uremia. A qualitative platelet defect. Lancet. 1966;2(7454):66–69.
- Nakamura Y, Tomura S, Tachibana K, Chida Y, Marumo F. Enhanced fibrinolytic activity during the course of hemodialysis. Clin Nephrol. 1992;38(2):90–96.
- Sreedhara R, Itagaki I, Hakim RM. Uremic patients have decreased shear-induced platelet aggregation mediated by decreased availability of glycoprotein IIb-IIIa receptors. Am J Kidney Dis. 1996;27(3):355–364.