Abstract
Background: Experience with hydroxyethyl starch (HES) in children is limited. This study was conducted to observe the effects of HES or Ringer’s lactate (RL) usage as the priming solution on renal functions in children undergoing cardiac surgery. Methods: After ethical committee approval and parent informed consent, 24 patients were included in this prospective, randomized study. During cardiopulmonary bypass (CPB), Group I received RL and Group II received HES (130/0.4) as priming solution. Serum creatinine, blood urea nitrogen (BUN), β2-microglobulin, cystatin C, and urinary albumin and creatinine, serum, and urine electrolytes were analyzed after the induction (T1), before CPB (T2), during CPB (T3), after CPB (T4), at the end of the operation (T5), on 24th hour (T6), and on 48th hour postoperatively (T7). Fractional sodium excretion (FENa), urinary albumin/creatinine ratio, and creatinine clearance were calculated. Drainage, urine output, inotropes, diuretics, and blood requirements were recorded. Results: In both the groups, β2-microglobulin was decreased during CPB and cystatin C was decreased at T3,T4, and T5 periods (p < 0.05) and the levels remained within the normal range. Creatinine clearance did not differ in the HES group, but increased in the RL group (p < 0.05). Urine albumin/creatinine ratio was increased (p < 0.05) after CPB in the HES group, and it increased at T3, T4, and T5 in the RL group (p < 0.05). There were no differences in cystatin C, β2-microglobulin, FENa, urine albumin/creatinine ratio, creatinine clearance, total fluid amount, urine output, drainage, and inotropic and diuretic requirements between the groups. Conclusion: We conclude that usage of HES (130/0.4) did not have negative effects on renal function, and it can be used as a priming solution in pediatric patients undergoing cardiac surgery.
INTRODUCTION
Renal dysfunction is the most critical complication after the cardiac surgery and its occurrence is more common in surgeries with cardiopulmonary bypass (CPB). Besides subclinical and temporary renal damages, renal failures that require dialysis can emerge. Also, mortality rate related to hemodialysis is increased. Moreover, hospitalization period and medical costs tend to increase due to acute kidney injury (AKI) and dialysis.Citation1
In patients who underwent surgery with CPB, panendothelial damage related to systemic inflammatory response is observed frequently. As a consequence, protein loss, increased endothelial permeability, and interstitial edema can emerge. Fluid loss from intravascular area to interstitial area leads to hypovolemia. During CPB, renal blood flow and vascular resistance decrease by 30%.Citation1 Hypoperfusion is the main reason of renal failure, resulting in renal ischemia. Emboli, renal ischemia, reperfusion injury, and pigments are the main reasons of renal damages during CPB. Ischemia–reperfusion-dependent AKI and nephropathy, and circular arrest episodes, are thought to be related to decreased cardiac output in perioperative and prolonged bypass duration. Ischemia–reperfusion damage affects not only tubules and glomeruli, but also renal vessels.
In children, elevated perfusion rates are required due to increased metabolic requirements, which also show destructive effects on the structural blood components. Although the CPB technology is advancing, CPB circuits are too large for pediatric population, and these circuits lead to critical decrease in the structural components of blood, coagulation factors, and other plasma proteins. In newborns and children, oliguric renal failure after CPB is very common.Citation2
The purpose of colloid usage during CPB is to sustain osmotic pressure to avoid fluid loss from tissues and decrease fluid retention. Hydroxyethyl starch (HES) solution is widely used as intravascular volume dilator and priming solution in CPB in adult patients.Citation3 It has been claimed that AKI risk is increased during hypovolemia treatment with HES. Therefore, the effects of HES on kidney functions are still investigated.Citation4 Although low-molecular-weight HES (130/0.4) solution does not affect kidneys, its effects on children are yet to be investigated. In this study, we aimed to observe the effects of HES (130/0.4) usage, as a priming solution, on the renal functions in child patients undergoing open heart surgery.
MATERIALS AND METHODS
After Local Medical Research Ethics Committee approval and parent informed consent, 24 patients aged 2–16 years were included in this prospective, randomized study. Patients with preoperative serum creatinine value above 1.5 mg/dL, heart failure, liver failure [aspartate transaminase (AST) > 40 unit/L, alanine aminotransferase (ALT) > 40 unit/L], insulin-dependent diabetes mellitus (DM), history of cardiac surgery, allergies, corticosteroids, nonsteroidal anti-inflammatory drugs, and chronic furosemide usage were excluded from the study. Also, patients who were operated in emergency conditions were not included in the study.
A part of the patients received intravenous (IV) access and these patients were anesthetized with thiopental 5 mg/kg; the remaining patients received Sevoflurane anesthesia induction, and then IV access. Tracheal intubation was carried out after vecuronium bromide 0.1 mg/kg and fentanyl 2–3 μg/kg injection. Anesthesia maintenance was sustained by Sevoflurane 1.5–2% and 50% O2–50% air combinations. Electrocardiogram (ECG), partial oxygen saturation (SpO2), arterial blood pressure, central venous pressure (CVP), and urine output were monitored in all the patients.
The patients were divided into two randomized groups according to the priming solution used in the CPB: Group I and Group II. Each group included 12 patients. In Group I, RL solution was used as priming solution, whereas in Group II, HES (130/0.4) solution was used.
CPB was performed using modified De Bakey roller pump, membrane oxygenator (Dideco membrane D-901 or D-902 according to weight of the patient), nonpulsatile blood flow (2.4 mL/m2), and moderate hypothermia (28–32°C) and mean perfusion pressure was kept between 40 and 50 mmHg during CPB. Mannitol 20% 1 g/kg was added to the priming volume. Hematocrit level was kept above 24% for patients with weight >10 kg and 30% for patients with <10 kg. When the adequate hemodynamic were obtained, the patients were weaned from CPB. Heparin was neutralized with protamine. Erythrocyte suspension was applied to achieve hemoglobin level above 10 g/dL.
All the patients were administered to Cardiac Surgery Intensive Care Unit (ICU) after the operation. The patients with normal body temperature, normal hemodynamics, and controlled bleeding were separated from the mechanical ventilation.
Demographic and clinical data (age, sex, weight, operation duration, CPB duration, and aortic cross-clamping time), ICU duration, and hospitalization duration were recorded for all the patients.
Laboratory parameters of blood and urine samples were collected and evaluated after the induction (T1), before CPB (T2), on the 20th minute of CPB (T3), after CPB (T4), at the end of the operation (T5), on 24th hour (T6), and on 48th hour postoperatively (T7) in all patients. Hemodynamic parameters including systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), heart rate, and CVP were recorded at the same time except the CPB.
Blood samples were analyzed for hematocrit and serum electrolytes [sodium (Na), potassium (K), chloride (Cl)], creatinine, blood urea nitrogen (BUN), β2-microglobulin, and cystatin C levels at T1–T7. To determine cystatin C and β2-microglobulin levels, blood samples were centrifuged and kept in −20°C until the measurement time. Blood samples were then thawed in room temperature and cystatin C levels were measured using particle-enhanced immunoturbidimetric test, and β2-microglobulin levels were measured using two-phase chemiluminescent immunoassay test. Blood neutrophil levels were analyzed at T1, T5, T6, and T7 periods.
In the urine samples collected from the patients, the following urine electrolytes were monitored: Na, K, Cl, albumin, and creatinine. Fractional sodium excretion (FENa) value was determined by using the formula: (urine Na × plasma creatinine × 100)/(urine creatinine × plasma Na) at T1–T7 periods. Urine albumin/creatinine ratio was calculated to determine the glomerular damage at the same time. Creatinine clearance was determined at T1, T5, T6, and T7 periods according to the following formula: Cr. Clearance (mL/min) = [(140 – age) × (body weight)]/[serum creatinine (mg/dL) × 72].
Total fluid administration, urine output, fresh frozen plasma and erythrocyte suspension amount, inotropic and diuretic requirements, and drainage amount from chest tube were recorded at the end of the operation, on 24th and 48th hours postoperatively, in all the patients. The first stage of AKI was defined by at least one of the following criteria: an increase in serum creatinine of at least 50% above baseline, an absolute increase in serum creatinine of at least 0.3 mg/dL, and/or a urine output <0.5 mL/kg/h for at least 6 hours.
Statistical Analysis
The statistical analysis of the data was processed by SPSS 15.0 program. Shapiro–Wilk test was used to determine the distribution pattern of the data. t Test was used for normally distributed data, and Mann–Whitney U test was used for independently distributed data to compare the two groups. Friedman two-way analysis of variance test was used to compare the time parameters of both the groups. In the case of difference, Wilcoxon signed-rank test was applied to compare the time parameters of both the groups. Chi-square test was applied to analyze the categorical data. Significance level was set to p < 0.05.
RESULTS
Twenty four patients were included in the study. There were no differences between the groups regarding age, gender, aortic cross-clamping time, operation, CPB, ICU duration, and hospitalization time (). There was no difference in the SAP, DAP, MAP, and CVP records between the groups. Only the heart rate was found to be increased (p < 0.05) in the RL group at T4 and at the end of the operation (T5).
Table 1. Demographic and clinical data of Groups I and II (mean ± SD).
Serum and urine electrolyte levels also did not differ significantly between the groups. β2-Microglobulin levels were found to be decreased at T3 in both the groups when compared with T1 (p < 0.05). There were no differences between the two groups ().
Table 2. β2-Microglobulin levels (mean ± SD) (ng/dL).
Cystatin C levels were decreased in both the groups at T3, T4, and T5 compared with basal levels (p < 0.05) (). All the determined β2-microglobulin and cystatin C levels were in the normal range. The normal β2-microglobulin levels were in the range 1010–1730 ng/dLCitation5 and the normal cystatin C levels were specified between 0.63 and 1.33 mg/L in children (1–16 years old).Citation6
Table 3. Cystatin C levels of the RL and HES groups (mean ± SD) (mg/L).
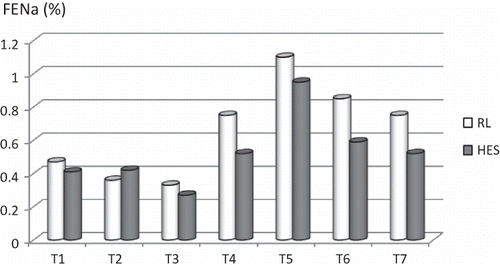
Serum creatinine, BUN, urine albumin, and urine creatinine levels did not differ significantly between the groups. Calculated FENa levels did not differ between the RL and HES groups ().
Figure 2. Creatinine clearance levels in the RL and HES groups. Note: *p < 0.05 within the group, compared to (T1) period.
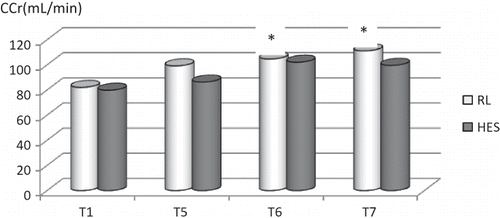
While creatinine clearance values did not differ in the HES group, they were increased significantly in the RL group on 24th and 48th hours postoperatively compared with the T1 (p < 0.05). There were no differences between the groups ().
While Urine albumin/creatinine ratio was increased significantly (p < 0.05) only after CPB in the HES group, significant increase was observed during CPB too at the end of the operation (T3, T4, and T5) in the RL group (p < 0.05) when compared with basal level. The ratio did not differ significantly between the groups ().
Table 4. Urine albumin/creatinine ratios in patients (mean ± SD) (μg/mg).
Total fluid administration, total urine output, drainage amount from chest tube and erythrocyte suspension (ES) and fresh frozen plasma (FFP) support did not differ significantly between the two groups (p > 0.05), ().
Table 5. Fluid balance of Groups I and II (mean ± SD) (×103/μL).
Diuretic (furosemide) was applied to four patients in the HES group and five patients in the RL group. Diuretic usage statistics did not differ significantly between the groups (p > 0.05).
Inotropic support (dopamine) was required for two patients in the HES group and two patients in the RL group. Inotropic requirements did not differ significantly between the groups.
Neutrophil levels were increased at the end of the operation (T5) and still higher on the 48th hour postoperatively (T6, T7) compared to the basal levels (T1) in both within the groups (p < 0.05). The comparison level of the two groups did not show significant difference when compared between the two groups (p > 0.05)().
Table 6. Neutrophil levels in the RL and HES groups (mean ± SD) (×103/μL).
Table 7. ES and RL/HES amounts added into priming solution (mean ± SD) (mL).
As the priming solution, 391,417 ± 207,25 mL RL was used in the RL group and 316,67 ± 86,164 mL HES (130/0.4) in the HES group ().
We did not observe any renal dysfunction (an increase in serum creatinine of at least 50% above baseline, an absolute increase in serum creatinine of at least 0.3 mg/dL, and/or a urine output <0.5 mL/kg/h for at least 6 hours) in both the groups.
DISCUSSION
We evaluated HES (130/0.4) usage as priming solution on subclinical and clinical renal functions on pediatric patients undergoing cardiac surgery. We compared HES and RL solutions. Both the groups were similar regarding to demographic and clinical data.
In pediatric age group, acute renal failure (ARF) was the most commonly observed complication of CPB and showed poor prognosis.Citation7 In a study conducted by Chesney and colleagues,Citation8 among 248 infant patients, 20 patients developed ARF, 6 patients required dialysis, and 13 patients were lost. In another study conducted by Ridgen and colleagues,Citation9 the risk factors that lead to ARF development after CPB were investigated in pediatric patients. The research team concluded that complexity of cardiac diseases, risky nature of cardiac surgery and long CPB period, chronic hypoxia and acidosis as the result of already existing diseases, and heart failure observed in early terms increased the incidence of postoperative ARF. The group reported a significant increase in ARF ratio, especially in newborn patients. It was also stated that in pediatric patients, already existing diseases and age were more crucial parameters for determining the postoperative renal failure than bypass time period alone.
In our study, none of the patients developed renal failure or required dialysis.
During CPB, the contact between the blood components and synthetic surfaces released the inflammatory mediators, which led to endothelial permeability increase.Citation10–12 Furthermore, several changes were observed in intravascular volume status and intravascular fluid during the bypass.Citation13,14 One of the disadvantages of the hemodilution is that it decreases the intravascular osmotic pressure and leads to interstitial edema formation. Especially, the crystalloid fluids can increase the extracellular fluid volume and cause massive interstitial fluid volume expansion.
Colloids are able to stay in the intravascular compartment for a long time and sustain normovolemia with small amounts. Thus, they do not cause excess fluid volume.Citation15 Therefore, to provide osmotic pressure, and reduce the fluid escape to tissue and fluid retention, fluids that have high oncotic pressure (HES, albumin, and gelatin) were selected as the priming solutions. These solutions can prevent capillary escape, which leads to fluid escape to tissue intervals, and tissue edema, which is caused by decreased oncotic pressure during CPB.Citation16,17
Several studies that used albumin and HES as priming solutions indicate that conservation of colloid osmotic pressure during CPB reduced the fluid amount accumulated between the tissues.Citation18 Also, colloidal priming solution usage reduced the total blood volume and intraoperative fluid requirement.Citation17
Tiryakioglu and colleaguesCitation19 compared the RL and HES 130/0.4 usage as the priming solution on adult patients and observed that BUN and serum creatinine levels were higher in the HES 130/0.4 group at postoperative 24th hour. The research group evaluated the possible negative effects of HES 130/0.4 on renal functions, but serious renal damages or failures were not observed in patients. Even though the urea and creatinine levels were higher compared with the control group, the levels were in the normal range. Also, serum electrolyte levels such as Na, K, Cl, and Ca did not differ significantly between the groups.
In our study, we did not find significant differences between the creatinine levels of both the groups. Also, urine and serum electrolytes did not show significant difference between the groups.
Ooi and colleaguesCitation20 conducted a research on 90 adult patients who underwent coronary artery bypass graft surgery. Forty-five patients received 6% HES 130/0.4 (Voluven) and another 45 were given 4% succinylated gelatin (Gelofusine) as the priming solution for the CPB circuit as well as for volume replacement. They compared the coagulation parameters and changes in the renal functions. The study demonstrated that HES 130/0.4 did not have negative effects on renal functions and coagulation parameters. It was concluded that 6% HES130/0.4 is a safe alternative colloid for priming the CPB circuit and volume replacement in patients undergoing coronary artery bypass surgery.
Cystatin C is a cysteine protease inhibitor that is synthesized by nucleated cells and subsequently released in the blood stream, especially after renal injury. Recent trials suggested that cystatin C has clinical utility in the early detection of cardiac surgery associated AKI and it was superior to serum creatinine.Citation21
Herrero-Morin and colleaguesCitation5 performed a study to identify the renal failures by comparing serum creatinine to cystatin and β2-microglobulin on child patients administered to ICU. Serum cystatin C and β2-microglobulin are more reliable and convenient markers than creatinine to identify ARF and minor differences in glomerular filtration rate (GFR). Unlike the other biomarkers, cystatin C is not affected by inflammatory, immunologic, and neoplastic disturbances.Citation22 Also, because of the difficulties during urine sample collection from children and urine loss during this procedure, cystatin C is accepted to be a better biomarker than serum creatinine to determine the renal function progresses in pediatric population.Citation5 In pediatric population, cystatin C concentration levels are elevated after birth, but started to decrease quickly in the following weeks. In children between 1 and 16 years of age, the normal range of cystatin C is determined as 0.63–1.33 mg/L.Citation5,6 Therefore, we monitored cystatin C and β2-microglobulin levels in our study.
In our study, the levels of cystatin C were significantly decreased during CPB, after CPB, and after the operation in both the groups. We observed that measured cystatin C levels remained within normal range for all the study periods in both the groups.
When we examined β2-microglobulin levels, we observed that β2-microglobulin levels were found to be decreased during CPB in both the groups when compared with baseline. There were no differences between the two groups and all the levels were within the normal range. Since β2-microglobulin levels did not increase and remained within the normal range, we considered that there was no renal tubular damage.
During CPB, neutrophils and vascular endothelium were shown to be activated via upregulation of adhesion molecules such as CD11b and CD4 and thought to play an important role on pathophysiology of renal complications.Citation23,24 HES solution can decrease vascular permeability and inflammatory response activation during CPB by preventing leukocyte adhesion and chemotaxis.Citation25 In our study, increase in both the groups was detected as expected. However, there were no significant differences between the groups.
Haneda and colleaguesCitation26 claimed that colloidal solutions should be added to priming solutions in treatment of pediatric patients. In their retrospective study, they demonstrated that fluid balance of colloid hemodilution was superior to crystalloid hemodilution.
Aukerman and colleaguesCitation27 demonstrated that albumin addition to priming solution in pediatric patients led to less increase in the off-pump weight. Another study with albumin conducted by Riegger and colleaguesCitation28 demonstrated that off-pump weight was similar. However, blood transfusion requirement was increased.
Loeffelbein and colleaguesCitation29 examined the effects of albumin in priming solutions and impact of FFP on renal functions after CPB, by evaluating protein excretion in urine, creatinine, and creatinine clearance in newborns and infants. As the result, intraoperative renal functions were found to be distorted in both the groups but not in the significant levels. Also, protein loss in postoperative and creatinine clearance levels did not differ significantly.
When the intraoperative and postoperative urine samples were examined, no significant differences were found in microalbumin, creatinine, and creatinine clearance levels. Furthermore, we determined urinary albumin/creatinine ratio to assess glomerular damage. Even though the two groups did not show significant difference, we determined a significant increase within the levels of the RL group during CPB and it increased after operation and it was decreased on postoperative 24th hours. Albumin/creatinine (Alb/Cr) ratio was increased only after CPB in the HES group. But, there was no any difference between the two groups regarding elevated levels of FENa and any changes were not determined in the levels of β2-microglobulin in the same time intervals. Therefore, these findings suggested that only an increase in Alb/Cr ratio levels is not sufficient for the diagnosis of transient tubulointerstitium damage. We consider glomerular edema related to interstitial volume increase and nephron ischemia as a reason.
In our study, inotropic (dopamine) and diuretic (furosemide) usage did not differ significantly in the two groups.
The studies in the literature that was based on HES 130/0.4 usage in CPB to understand its effects on renal functions were based on adult patients. In those studies, it was found that HES 130/0.4 usage as the priming solution was reliable. We used HES 130/0.4 solution as priming solution in pediatric CPB patients and tried to examine the effects on renal functions. In both the groups, we did not detect any renal functional difference. We also did not observe any negative effects of HES 130/0.4 on kidney functions.
We conclude that HES 130/0.4 usage as the pump priming solutions in pediatric patients who are undergoing cardiac surgery did not show any negative effects on renal functions and it can be used safely as the CPB priming solution in pediatric patients undergoing cardiac surgery.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was financially supported by the Department of Anesthesiology and Reanimation, Hacettepe University, Faculty of Medicine.
REFERENCES
- Arora P, Kolli H, Nainani N, Nader N, Lohr J. Preventable risk factors for acute kidney injury in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2012;26(4):687–697. Epub 2012 Apr 18.
- Davies LK. Cardiopulmonary bypass in infants and children: how is it different? J Cardiothorac Vasc Anesth. 1999;13(3): 330–345.
- Chong Sung K, Kum Suk P, Mi Ja Y, Kyoung Ok K. Effects of intravascular volume therapy using hydroxyethyl starch (130/0.4) on post-operative bleeding and transfusion requirements in children undergoing cardiac surgery: a randomized clinical trial. Acta Anaesthesiol Scand. 2006;50(1):108–111.
- Wyckoff T, Augoustides JG. Advances in acute kidney injury associated with cardiac surgery: the unfolding revolution in early detection. J Cardiothorac Vasc Anesth. 2012;26(2):340–345.
- Herrero-Morin JD, Malaga S, Fernandez N. Cystatin C and beta2-microglobulin: markers of glomerular filtration in critically ill children. Crit Care. 2007;11(3):R59.
- Finney H, Newman DJ, Thakkar H, Fell JM, Price CP. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000;82(1):71–75.
- Ogg CS, Cameron JS. Cardiovascular surgery and the kidney. Guys Hosp Rep. 1969;118(1):85–103.
- Chesney RW, Kaplan BS, Freedom RM. Acute renal failure: an important complication of cardiac surgery in infants. J Pediatr. 1975;87(3):381–388.
- Rigden SP, Barrat TM, Dillon MJ. Acute renal failure complicating cardiopulmonary bypass surgery. Arch Dis Child. 1982;57(6):425–430.
- Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112(3):676–692.
- Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75(2):S715–S720.
- Chenoweth DE, Cooper SW, Hugli TE. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304(9):497–503.
- Mehlhorn U, Davis KL, Burke EJ. Impact of cardiopulmonary bypass and cardioplegic arrest on myocardial lymphatic function. Am J Physiol. 1995;268(1 Pt 2):H178–H183.
- Heltne JK, Bert J, Lund T. Temperature-related fluid extravasation during cardiopulmonary bypass: an analysis of filtration coefficients and transcapillary pressures. Acta Anaesthesiol Scand. 2002;46(1):51–56.
- Base EM, Standl T, Lassnigg A, . Efficacy and safety of hydroxyethyl starch 6% 130/0.4 in a balanced electrolyte solution (Volulyte) during cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25(3):407–414.
- Kuitunen AH, Hynynen MJ, Vahtera E. Hydroxyethyl starch as a priming solution for cardiopulmonary bypass impairs hemostasis after cardiac surgery. Anesth Analg. 2004;98(2):291–297.
- Rex S, Scholz M, Weyland A. Intra- and extravascular volume status in patients undergoing mitral valve replacement: crystalloid vs. colloid priming of cardiopulmonary bypass. Eur J Anaesthesiol. 2006;23(1):1–9.
- Tollofsrud S, Svennevig JL, Breivik H. Fluid balance and pulmonary functions during and after coronary artery bypass surgery: ringer’s acetate compared with dextran, polygeline, or albumin. Acta Anaesthesiol Scand. 1995;39(5):671–677.
- Tiryakioglu O, Yıldız G, Vural H. Hydroxyethyl starch versus Ringer solution in cardiopulmonary bypass prime solutions (a randomized controlled trial). J Cardiothorac Surg. 2008;3:45.
- Ooi JS, Ramzisham AR, Zamrin MD. Is 6% hydroxyethyl starch 130/0.4 safe in coronary artery bypass graft surgery? Asian Cardiovasc Thorac Ann. 2009;17(4):368–372.
- Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatininee as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226.
- NewmaN DJ, Thakkar H, Edwards RG. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatininee. Kidney Int. 1995;47(1):312–318.
- Galinanes M, Watson C, Trivedi U. Differential patterns of neutrophil adhesion molecules during cardiopulmonary bypass in humans. Circulation. 1996;94(Suppl. 9):II364–II369.
- Rinder CS, Fontes M, Mathew JP. Neutrophil CD11b upregulation during cardiopulmonary bypass is associated with postoperative renal injury. Ann Thorac Surg. 2003;75(3):899–905.
- Iriz E, Kolbakır F, Akar H. Comparison of hydroxyethyl starch and ringer lactate as a prime solution regarding S-100beta protein levels and informative cognitive tests in cerebral injury. Ann Thorac Surg. 2005;79(2):666–671.
- Haneda K, Sato S, Ishizawa E. The importance of colloid osmotic pressure during open heart surgery in infants. Tohoku J Exp Med. 1985;147(1):65–71.
- Aukerman J, Vopel-Lewis T. The relationship between extracorporeal circuit prime, albumin, and postoperative weight gain in children. J Cardiothorac Vasc Anesth. 1998;12(4):408–414.
- Riegger LQ, Voeppel-Lewis T, Kulik TJ. Albumin versus crystalloid prime solution for cardiopulmonary bypass in young children. Crit Care Med. 2002;30(12):2649–2654.
- Loeffelbein F, Zirell U, Benk C. High colloid oncotic pressure priming of cardiopulmonary bypass in neonates and infants: implications on hemofiltration, weight gain and renal function. Eur J Cardiothorac Surg. 2008;34(3):648–652.