Abstract
Data on routine use of continuous erythropoietin receptor activator (CERA) in peritoneal dialysis patients are scarce. This study aimed to assess the efficacy of CERA administered once monthly in maintaining stable Hb levels in patients on peritoneal dialysis under routine medical practice. This was a 12-month, observational, prospective and multicenter study. A total of 83 patients with anemia secondary to chronic kidney disease (CKD) on peritoneal dialysis for more than 3 months, on once-monthly subcutaneous CERA treatment, were followed up over a period of 1 year. Efficacy evaluation included Hb levels, mean time in which the Hb level was maintained within target range, CERA doses and number of dose changes. Median Hb level (interquartile range [IQR]) remained stable during the evaluation period [11.8 ± 1.4 g/dL at baseline, 11.8 ± 1.4 g/dL at month 6 and 11.8 ± 1.5 g/dL at month 12 (p > 0.05)]. The median (IQR) time of Hb level maintained within target range (11–13 mg/dL) was 6 (4–10) months. Ferritin, transferrin saturation index, and Fe were also stable and well maintained during the 12 months (p > 0.05). CERA mean dose (SD) was [115.4 (56.2) μg baseline; 117.2 (58.5) μg 6 months; 126.0 (65.9) μg 12 months (p = 0.127)]. The mean number of CERA dose changes per patient during the study was 1.6 (SD 1.3). Serious adverse events were not related to CERA treatment. The results suggest that once-monthly CERA successfully corrects anemia and maintains stable Hb levels within the recommended target range on peritoneal dialysis under routine medical practice.
INTRODUCTION
Anemia, due to the decrease in erythropoiesis-stimulating agent (ESA) synthesis by the damaged kidney, is a common condition in patients with chronic kidney disease (CKD), and contributes to morbidity and mortalityCitation1,2 (mainly of cardiovascular origin) and reduces the quality of life in these patients.Citation3,4
As in other groups of CKD patients, the treatment of anemia is of paramount importance in the general management of patients receiving regular peritoneal dialysis.Citation5 Treatment with conventional ESA to manage anemia in CKD patients needs frequent administrations, changes of doses, and close monitoring of hemoglobin (Hb) concentrations.Citation6 Increasing the administration intervals of ESAs represents an opportunity to improve the efficiency of anemia management in patients with CKDCitation7 and to ameliorate the quality of life of patients through saving frequent punctures.
A first continuous erythropoietin receptor activator (CERA) methoxy polyethylene glycol-epoetin beta (Mircera®) provides correction of anemia and stable control of Hb levels at extended administration intervalsCitation4,8,9 with a unique pharmacologic profile and a long half-life (139 h after subcutaneous administration in patients who are on peritoneal dialysis.Citation10 Numerous randomized Phase III trials demonstrated that CERA administered once every 4 weeks effectively maintains Hb levels stable in patients with CKD on dialysis Citation4,6,11 and offers similarCitation6,12 or superior Citation11efficacy compared with shorter-acting ESAs as maintenance therapy. Recent studies have also proved that dialysis patients can be successfully converted from epoetin or darbepoetin-α to monthly CERA maintenance therapy.Citation4,7,13,14 However, only a low proportion of those patients were on peritoneal dialysis. As a consequence, these randomized studies do not provide real evidence concerning the efficacy and safety of the use of CERA in patients on peritoneal dialysis in day-to-day clinical practice. Few trials have analyzed CERA efficacy exclusively in peritoneal dialysis patients,Citation10,15–17 and few of them had been conducted in routine clinical conditions.Citation17 Noninterventional studies may provide complementary information on the effectiveness of CERA in real-life conditions. The aim of this study was to evaluate the efficacy of once-monthly CERA administration maintaining stable Hb levels in CKD patients on peritoneal dialysis, under routine medical practice.
PATIENTS AND METHODS
We have performed a 12-month, observational, prospective and multicenter study under routine medical practice, in Catalonia, an Autonomous Community of Spain. The study was conducted in 112 adult patients (≥18 year of age) with anemia secondary to CKD who had been receiving peritoneal dialysis for more than 3 months and treated with once-monthly subcutaneous CERA [Mircera® (methoxy polyethylene glycol-epoetin beta); F. Hoffmann-La Roche Ltd., Basel, Switzerland], at least 1 month before to start data collection.
This nonintervention nature study was carried out between September 2008 and December 2009, with patients from eight Catalan Hospitals and was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization of Good Clinical Practice guidelines and local Ethics Committees. All study participants provided their written informed consent to collect clinical data.
CERA was dosed according to the decision of the each clinician and not given by protocol. However, a target of Hb level was established within the range of international guidelines.Citation18,19 Patients were monitored once every 2 months. Results from basal, 6 months, and 12 months are shown.
Cause of CKD, doses of prior ESAs administered, starting dose of CERA and types of peritoneal dialysis were recorded. Systolic blood pressure (SBP) and diastolic blood pressure (DBP), adequacy of dialysis (Kt/V), and laboratory parameters as transferrin saturation index (TSI), Hb, iron (Fe) status, ferritin, B12 vitamin, C-reactive protein (CRP), albumin, cholesterol, and doses of CERA were assessed throughout the study. Hb was considered to have remained stable when the Hb level was within the Hb target range (11–13 g/dL)Citation18,19 at consecutive visits.
Efficacy assessment included Hb levels, the average of patients who reached the target Hb (11–13 g/dL),Citation18,19 the mean number of CERA dose modifications per patient during the study, the proportion of patients who maintain the Hb level within the target range and mean time in which the Hb level was maintained within this target range. Serious adverse events information was collected throughout the whole observation period.
Patient characteristics were described by means and standard deviations for quantitative variables and by the distributions of absolute and relative frequencies for qualitative variables. Tests were two-tailed with a significance level of 5%. Data were analyzed using SAS statistical software. To determine possible relationships, Spearman’s rho test was calculated between the CERA dose, Hb level, and clinical parameters.
RESULTS
Study Population
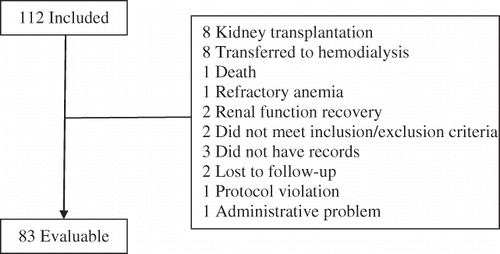
A total of 112 patients were included in the study but only 83 (74.1%) of these remained over the whole study period and were evaluable. The reasons for premature withdrawal and/or exclusion from the analysis of the remaining 29 (25.9%) patients were kidney transplantation (n = 8), transfer to hemodialysis (n = 8), death (n = 1), refractory anemia (n = 1), renal function recovery (n = 2), did not meet inclusion/exclusion criteria (n = 2), did not have Hb records (n = 3), lost to follow-up (n = 2), protocol violation (n = 1), and administrative problem (n = 1) ().
Patient characteristics at baseline and the etiology of the renal disease are summarized in . The majority of patients were men (59.0%); the mean age was 57.8 years. Glomerulonephritis was the main cause of CKD (24.1%); 59 (71.1%) patients received peritoneal dialysis as first treatment option, 12 (14.5%) had previously received hemodialysis treatment, and 12 (14.5%) had kidney graft failure and returned to dialysis.
Table 1. Clinical and sociodemographic characteristics.
Almost all patients (89.1%) had previously received other ESA therapy before CERA treatment [53 (71.62%) darbepoetin-α (42.5 ± 24.3 μg/week), 19(25.6%) epoetin-β (7105 ± 5849 IU/week), and 2 (2.7%) epoetin alfa (5000 IU/week and 18,000 IU/week)], and 9 (10.84%) were ESA-naïve patients.
The starting doses of CERA, in patients on previous ESA treatment or naïve patients, are shown in . ESA-naïve patients were initially treated with CERA mean doses of 87.5 μg/month, and 59% of the overall patients, who were on previous ESA dose of <8000 IU/week epoetin or <40 μg/week darbepoetin-α, were initially treated with a monthly CERA median dose of 100 μg. Likewise, patients with previous higher ESA dose categories (28.9%) were started with CERA doses of 150 and 200μg/month, all median CERA doses were lower than those recommended in the summary of product characteristic (SPC).
Table 2. Starting CERA doses according to previous ESA treatment.
At baseline, 52 (62.7%) patients were receiving automated peritoneal dialysis (APD), 26 (31.3%) continuous ambulatory peritoneal dialysis (CAPD), and 5 (6.0%) nighttime intermittent peritoneal dialysis (NIPD).
Efficacy Evaluation
shows the evolution of the anemia parameters. Statistically significant differences were not observed in the course of the 12-month study. The mean Hb levels in ESA naïve patients remained stable and within the recommended target range during the study, 11.8 ± 1.9 g/dL (p = 0.309). Patients converted from other ESA also presented stable Hb levels within the target range, irrespective of the frequency of administration of previous ESAs, 11.8 ± 1.5 g/dL (p = 0.622). In the total study population, the mean and median Hb levels were well maintained at nearly 12 g/dL during the evaluation period (p = 0.780) ().
Table 3. Evolution of the anemia parameters.
With regard to the average of patients within the target Hb range (11–13 g/dL), at the different visits were shown not statistically significant differences (59.0%, baseline; 56.6%, 6 months; 44.6%, 12 months; p = 0.323).
The median [interquartile range (IQR)] time in which the Hb level was maintained within the target range (11–13 mg/dL) was 6 months.Citation4–10 About 45.8% of the patients succeeded in maintaining their Hb levels within 11–13 g/dL during 8 months or more than 8 months, and 10% of the patients maintained it throughout the 12 months of the study.
B12 vitamin, Fe, Ferritin, and TSI were stable and well maintained during the 12 months (p > 0.05, ).
Monthly CERA mean dose did not show statistically significant differences in the course of the 12-month study neither in the total study population nor in naïve or treated previously with other ESAs patients (). We observed that monthly CERA dose employed was lower than those recommended in the SPC ().
Table 4. Evolution of CERA doses.
The mean number of CERA dose changes per patient in the overall study population during the evaluation period was 1.6 (SD 1.3) and per naïve patients 1.0 (SD 0.89).
The evolution of characteristics of peritoneal dialysis and cardiovascular clinical parameters are summarized in . The mean dose of dialysis administered (Kt/V) was similar and within normal values during the whole evaluation period, as well as blood pressure and cholesterol levels (p > 0.05, ). Just the microinflammatory state measured by CRP showed an improvement (p < 0.05, ). There was no relation between Kt/V, cholesterol, albumin, or CRP levels and CERA doses or Hb levels at any visit (Spearman’s rho correlation coefficient was <0.3).
Table 5. Evolution of analytical data and blood pressure.
Safety
Severe adverse events were reported in 19/83 patients (22.89%) during the 12-month treatment phase. Out of the 26 serious adverse events reported in these 19 patients, 7 were related to CKD (6 infectious diseases and 1 metabolism and nutrition disorder). One patient died during the 12-month study. Death was from an unknown cause. SAEs were not related to CERA treatment.
DISCUSSION
ESAs are necessary in most CKD patients on dialysis to maintain Hb levels, preventing anemia complications and ameliorating both the quality and the expectancy of life. Different ESAs have been used in the last few years. CERA has been demonstrated effective in maintaining stable Hb levels by once-monthly administration in dialysis patients with CKD in numerous randomized studies.Citation4,6,11 However, most of the subjects in those were on hemodialysis. Few trials have analyzed CERA efficacy exclusively in peritoneal dialysis patients,Citation10,15–17 and few of them were conducted in routine clinical conditions.Citation17
Results from this observational study indicate that once-monthly CERA maintains stable levels of Hb within the recommended target range, under routine clinical conditions, in patients on peritoneal dialysis. During the 12-month evaluation period, Hb levels remained stable, with a low number of CERA dose changes.
The mean number of CERA dose changes during the study was 1.6 in a 12-month period. This result was higher than reported in a historical cohort studyCitation17; the reason may be the presence of näive patients in a titration period in our study. Previous studies conducted in hemodialysis patients reported a mean of CERA dose change of nearly 2.Citation12,14
Dose changes have been related to fluctuations in Hb. Several studies have reported that Hb levels frequently fluctuate over time in patients treated with ESAs.Citation17,20–23 Fluctuation in serum Hb have been linked to poor patient outcomes.Citation22 The longer half life of CERA may offer a small advantage in reducing the degree of Hb variability, possibly because of fewer dose changes per patient.Citation17
Although Hb fluctuations are smaller in patients on peritoneal dialysis compared with those on hemodialysis,Citation12,13,22 our findings, as other authors have also reported,Citation12,13,17 underline the difficulty of maintaining Hb levels within the narrow range of the guideline recommendations over long periods of time. Thus, the mean time of maintained stable Hb levels within range (11–13 g/dL) in our study was 6.3 months. In the historical cohort study in peritoneal patients, above mentioned,Citation17 31.6% of patients in the CERA group had stable serum Hb with no fluctuations during the 12 months, but only 26 patients were treated with CERA. In our study, 10% of the patients succeeded in maintaining Hb levels within the target range throughout the study. Different authors had estimated that only 5% of patients who are on hemodialysis have Hb between 11–12 g/dL persistently remain within that range for 6 months.Citation4,24
Monthly administration of CERA is becoming more widely adopted,Citation14 so it is important to assess whether the efficacy observed in the controlled trial setting is matched when dosing is adjusted at the clinician’s prescription. Due to the nonintervention nature of this study, treatment patterns were entirely according to the physician’s clinical judgment. The required doses are lower in peritoneal dialysis than in hemodialysis patients due to better adequacy of dialysis and lower hematological losses in the capillary filters. In clinical practice, lower and better stratification of the initial CERA dosage has been proved compared with the doses recommended in the SPC.Citation25 The information provided by the present study suggests that CERA is effective even at dose levels lower than the dosage recommended by SPC. So, mean Hb levels remained close to 12 g/dL over the whole evaluation period. Regarding this issue, the prescription of CERA doses below recommended values has been reported in several studiesCitation13,25 and it was considered effective in maintaining Hb levels in CKD patients not on dialysis.Citation25
In peritoneal dialysis patients, the need for frequent punctures could diminish their quality of life and increase the loss of adherence to treatment. The prolonged half-life of CERA could be beneficial in preventing these problems in peritoneal dialysis patients. ESAs are administered commonly via subcutaneous injections in patients on peritoneal dialysis, and different authors have reported that subcutaneous administration of CERA is significantly less painful than darbepoetin-α.Citation26 Therefore, CERA offers the advantage of monthly dosing, which provides a lower pain burden, avoids repeated controls at the hospital, and may improve patient compliance.Citation15
Some limitations should be borne in mind. Among them, the observational design of the study implies potential confounding factors. One of these was that the treatment pattern was entirely according to the physician’s clinical judgment and not determined by protocol. So, the decisions to initiate CERA, the initial dose, and subsequent dose adjustments were made at the discretion of the physician. It is therefore not certain that the treatment pattern was the same for all patients. Furthermore, this study included patients treated with once-monthly subcutaneous CERA during at least 1 month prior to start of data collection, so some patients could be still in the period of stabilization of their Hb levels during the first months of the study.
Randomized clinical trials employ restrictive criteria. However, these conditions do not reflect conditions in actual clinical practice. Observational studies assess the use of a drug in real conditions, providing complementary data. The information provided by the present study suggests that CERA is effective, even at dose levels lower than the dosage recommended by SPC, and maintains stable Hb levels within the recommended target range in peritoneal dialysis under routine medical practice conditions.
In conclusion, the fact that mean Hb levels were maintained stable over 1 year with a monthly dose of CERA, allows us to conclude that CERA may be an effective treatment of anemia in patients with end-stage renal failure on chronic peritoneal dialysis treatment in routine clinical practice.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was sponsored by Sociedad Catalano-Balear de Dialisis Peritoneal, with a financial support from Roche, Spain.
ACKNOWLEDGMENT
Editorial Assistance was provided by Content Ed Net.
REFERENCES
- Li S, Foley RN, Collins AJ. Anemia, hospitalization, and mortality in patients receiving peritoneal dialysis in the United States. Kidney Int. 2004;65:1864–1869.
- Horl WH. Anemia and its treatment in peritoneal dialysis patients. Wien Klin Wochenschr. 2005;117(Suppl 6):69–72.
- Perlman RL, Finkelstein FO, Liu L, . Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis. 2005;45:658–666.
- Sulowicz W, Locatelli F, Ryckelynck JP, . Once-monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J Am Soc Nephrol. 2007;2:637–646.
- Macdougall IC. How to optimize anemia therapy in peritoneal dialysis patients. Contrib Nephrol. 2006;150:202–213.
- Levin NW, Fishbane S, Canedo FV, . Intravenous methoxy polyethylene glycol-epoetin beta for hemoglobin control in patients with chronic kidney disease who are on dialysis: a randomized non-inferiority trial (MAXIMA). Lancet. 2007;370:1415–1421.
- Canaud B, Mingardi G, Braun J, . Intravenous C.E.R.A. maintains stable hemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrol Dial Transplant. 2008; 23:3654–3661.
- Macdougall IC. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436–440.
- Locatelli F, Villa G, de Francisco AL, . Effect of a continuous erythropoietin receptor activator (C.E.R.A.) on stable hemoglobin in patients with CKD on dialysis: once monthly administration. Curr Med Res Opin. 2007;23:969–979.
- Macdougall IC, Robson R, Opatrna S, . Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:1211–1215.
- Carrera F, Lok CE, De FA, . Maintenance treatment of renal anaemia in hemodialysis patients with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa administered monthly: a randomized comparative trial. Nephrol Dial Transplant. 2010;25:4009–4017.
- Weinreich T, Leistikow F, Hartmann HG, . Monthly continuous erythropoietin receptor activator treatment maintains stable hemoglobin levels in routine clinical management of hemodialysis patients. Hemodial Int. 2012 ;16(1):11–19.
- Dellanna F, Winkler RE, Bozkurt F, . Dosing strategies for conversion of hemodialysis patients from short-acting erythropoiesis stimulating agents to once-monthly C.E.R.A.: experience from the MIRACEL study. Int J Clin Pract. 2011;65:64–72.
- Fliser D, Kleophas W, Dellanna F, . Evaluation of maintenance of stable hemoglobin levels in hemodialysis patients converting from epoetin or darbepoetin to monthly intravenous C.E.R.A.: the MIRACEL study. Curr Med Res Opin. 2010;26:1083–1089.
- Chen CY, Hsu HJ, Lin YY, Wu MS. Subcutaneous continuous erythropoietin receptor activator conversion provides practical advantages and potential convenience for peritoneal dialysis patients. Perit Dial Int. 2011;31:592–597.
- Cano F, Alarcon C, Azocar M, . Continuous EPO receptor activator therapy of anemia in children under peritoneal dialysis. Pediatr Nephrol. 2011;26(8):1303–1310.
- Selby NM, Fonseca SA, Fluck RJ, Taal MW. Hemoglobin variability with epoetin beta and continuous erythropoietin receptor activator in patients on peritoneal dialysis. Perit Dial Int. 2012;32:177–182.
- Locatelli F, Aljama P, Canaud B, . Target hemoglobin to aim for with erythropoiesis-stimulating agents: a position statement by ERBP following publication of the trial to reduce cardiovascular events with aranesp therapy (TREAT) study. Nephrol Dial Transplant. 2010;25:2846–2850.
- Anon. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47:S11–S145.
- Lacson EJr, Ofsthun N, Lazarus JM. Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am J Kidney Dis. 2003;41:111–124.
- Regidor DL, Kopple JD, Kovesdy CP, . Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191.
- van der Putten K, van der Baan FH, Schellekens H, Gaillard CA. Hemoglobin variability in patients with chronic kidney disease in the Netherlands. Int J Artif Organs. 2009;32:787–793.
- Walker R, Pussell BA. Fluctuations in hemoglobin levels in hemodialysis, pre-dialysis and peritoneal dialysis patients receiving epoetin alpha or darbepoetin alpha. Nephrology (Carlton). 2009;14:689–695.
- Collins AJ, Brenner RM, Ofman JJ, . Epoetin alfa use in patients with ESRD: an analysis of recent US prescribing patterns and hemoglobin outcomes. Am J Kidney Dis. 2005;46:481–488.
- Minutolo R, Zamboli P, Chiodini P, . Conversion of darbepoetin to low doses of CERA maintains hemoglobin levels in non-dialysis chronic kidney disease patients. Blood Purif. 2010;30:186–194.
- Pannier A, Jordan P, Dougherty FC, Bour F, Reigner B. Subcutaneous injection pain with C.E.R.A., a continuous erythropoietin receptor activator, compared with darbepoetin alfa. Curr Med Res Opin. 2007;23:3025–3032.