Abstract
CXCR1 (CKR-1), a receptor of IL-8, is expressed in various cells including neutrophils and monocytes, both of which play a major role in proliferating glomerular diseases. We investigated time-dependent expression of CXCR1 and the effect of single-dose cyclosporine A (CsA) treatment on this expression in experimental mesangioproliferative glomerulonephritis induced by anti-thymocyte serum (ATS). Wistar rats were divided into three groups. Group 1 (control, n = 24) received non-immune serum. Group 2 (nephritis, n = 24) received ATS. Group 3 (nephritis + CsA, n = 24) received ATS and CsA concomitantly. Kidneys from six rats in each group were removed at sixth hour, 3 days, 5 days, and 7 days. ATS induced proteinuria compared to controls (p < 0.001) and CsA precluded the development of proteinuria. Glomerular inflammation and mesangial proliferation were significantly higher in ATS group than control and CsA-treated rats (p < 0.001). ATS injection caused marked interstitial inflammation that was precluded by CsA (p < 0.001). CXCR1 was not expressed in control kidneys. However, ATS induced expression of CXCR1 in both glomeruli and tubulointerstitium. CsA treatment precluded CXCR1 expression in both glomeruli and tubulointerstitium only in the first 6 h. CXCR1 may contribute to inflammation in experimental mesangioproliferative glomerulonephritis. CsA may be beneficial by inhibiting CXCR1 expression and corresponding inflammation.
INTRODUCTION
Proliferation of glomerular cells and infiltration of glomeruli by inflammatory cells are hallmark of various forms of glomerulonephritis including mesangial proliferative glomerulonephritis.Citation1 Chemokines are members of a family of chemotactic cytokines that play central roles in the recruitment of specific leukocyte subsets to inflammation sites, thereby contributing inflammation.Citation2–6 Among them, IL-8 was the first leukocyte selective chemokine to be identified and its corresponding receptors were the first chemokine receptors to be defined.Citation7–9 IL-8 is a highly specific cytokine for neutrophils. We and others have shown its importance in the pathogenesis of various forms of human glomerulonephritis.Citation10,11 IL-8 signals through the chemokine receptors, CXCR1 and CXCR2.Citation7 Among them, CXCR1 (CKR-1) is a G-protein-coupled chemokine receptor, expressed in various cells including neutrophils and monocytes.Citation12 However, renal expression of CXCR1 in experimental model of mesangioproliferative glomerulonephritis has not been investigated yet.
Injection of anti-thymocyte serum (ATS) is a widely accepted model for mesangial proliferative glomerulonephritis.Citation13,14 Injection of ATS to experimental animals produces complement dependent, selective injury to mesangial cells, which subsequently leads to mesangioproliferative glomerulonephritis.Citation15 Pathogenesis of this disease is not fully understood, but mesangial cell injury leads to infiltration of inflammatory cells (neutrophils and mononuclear cells) into the glomeruli, which in turn produce several growth factors and cytokines leading to mesangial proliferation.Citation16
In the present study, we investigated time-dependent expression of CXCR1 in experimental model of mesangioproliferative glomerulonephritis and the effect of a single-dose cyclosporin A (CsA) treatment on this expression.
METHODS
Animals
Male Wistar rats weighing 100–200 g, obtained from Animal Laboratory of Hacettepe University, were used in this study. Animals were housed in communal cages with food and water available ad libitum, and exposed to a 12-h light/dark cycle with the room temperature maintained at 21°C. The procedures in this study were approved by Hacettepe University Animal Care and Use Ethics Committee.
Experimental Protocol
Animals were divided into three groups. Group 1 (control, n = 24) rats received 5 mL/kg non-immune serum intravenously. Group 2 (nephritis, n = 24) rats received 5 mL/kg anti-thymocyte serum (ATS, Dako Corporation) intravenously to induce mesangioproliferative glomerulonephritis. Group 3 (nephritis + CsA, n = 24) rats received 5 mL/kg ATS intravenously and concomitantly injected with 25 mg/ kg CsA (Sigma–Aldrich Chemical Company) intravenously. Group 1 and 2 rats received intravenous saline treatment in equal volume (∼0.1 mL) with CsA administered in group 3. Six hours, 3 days, 5 days, and 7 days after ATS or non-immune serum injection, kidneys of six rats from each group were removed under diethyl ether anesthesia for histopathological and immunohistochemical studies. Twenty-four-hour urine was started to be collected in each rat 24 h before the day of kidney removal to assess proteinuria by means of urinary protein and creatinine ratio.
Immunohistochemical and Histopathological Studies
Kidneys of the rats were removed at the determined time points and fixed in 10% formalin. Tissues were embedded in paraffin wax and 5-μm sections were stained with hematoxylin and eosin for assessment of severity of glomerulonephritis. Expression of CXCR1, PCNA, and degree of glomerular inflammation, mesangial proliferation, and interstitial inflammation were evaluated by a pathologist (ZA) without prior knowledge of the treatment group. CXCR1 staining was graded semiquantitatively as 0 = no staining, 1 = staining in 1–25% of cells, 2 = staining in 26–50% of cells, 3 = staining in 51–75% of cells, and 4 = staining in 76–100% of cells. PCNA staining was calculated as percentage of all cells either in the glomeruli or tubulointerstitium. Kidney sections were examined for glomerular inflammation, mesangial proliferation, and interstitial inflammation and each individual section was graded as 0 = negative, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe for respective histopathological parameter. Inflammation was evaluated as infiltration of mononuclear cells into the respective renal compartment.
CXCR1 antibody, PCNA antibody, and goat ImmunoCruz Staining System were obtained from Santa Cruz Biotechnology Incorporation. Immunostaining procedures were performed according to the manufacturer’s instructions.
Statistical Analysis
Data were expressed as mean ± SEM. All measurements and calculations were analyzed with two-way ANOVA and post-hoc Bonferroni multiple comparison test. For all data sets, p < 0.05 was accepted to be statistically significant.
RESULTS
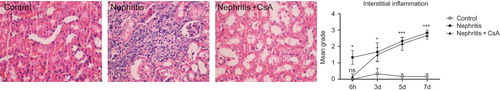
Urinary protein to creatinine ratio of control, nephritis and nephritis + CsA groups was presented in . ATS injection resulted in significant proteinuria compared to controls (p < 0.001), which persisted during the experiments. CsA treatment precluded the development of proteinuria in ATS injected rats (p < 0.001) during the experimental period. In parallel with proteinuria, glomerular inflammatory cells were significantly increased in nephritis (ATS) group compared to controls (, p < 0.001) and CsA treatment completely precluded infiltration of glomerular inflammatory cells in ATS-treated rats (p < 0.001).
Figure 1. Twenty-four hour urinary protein/creatinine ratio (mg/dL vs. mg/dL) of rats during the experimental period. Values are expressed as mean ± SEM. n = 6 at each time point, for each group.Notes: ***Denotes p < 0.001 against control and nephritis + CsA groups. CsA, cyclosporine A.
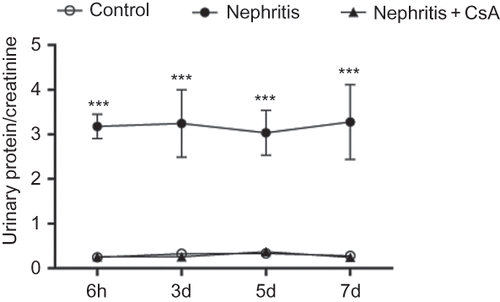
Figure 2. Glomerular inflammation in rat kidneys during the experimental period and histological appearances of kidneys in control (grade 0, at sixth hour), nephritis (grade 3, at sixth hour), and nephritis + CsA (grade 0, at sixth hour) groups. Values are expresed as mean ± SEM. n = 6 at each time point for each group. Notes: ***Denotes p < 0.001 against control and nephritis + CsA groups. CsA, cyclosporine A.
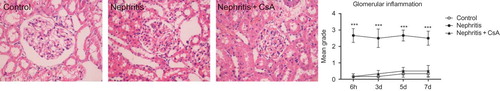
ATS resulted in severe mesangial proliferation starting in the first day after injection, reaching maximum around the fifth and seventh day (p < 0.001). CsA treatment had no effect on the development of mesangial proliferation ().
Figure 3. Mesangial proliferation in rat kidneys during the experimental period and histological appearances of kidneys in control (grade 0, on fifth day), nephritis (grade 4, on fifth day) and nephritis + CsA (grade 3, on fifth day) groups. Values are expressed as mean ± SEM. n = 6 at each time point for each group. Notes: ***Denotes p < 0.001 against control group. ns, not significant against control group. CsA, cyclosporine A.
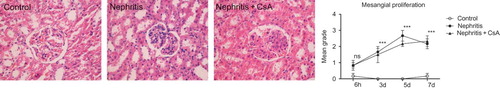
ATS injection caused marked renal interstitial inflammation, which started in the first day of post-injection period (, p < 0.05) and increased gradually (p < 0.001). CsA precluded the development of interstitial inflammation in the first day of post-injection period (p < 0.05), but on following days it had no effect on interstitial inflammation.
Figure 4. Interstitial inflammation in rat kidneys during the experimental period and histological appearances of kidneys in control (grade 0, at sixth hour), nephritis (grade 4, on seventh day), and nephritis + CsA (grade 0, at sixth hour) groups. Values are expressed as mean ± SEM. n = 6 at each time point for each group. Notes: *Denotes p < 0.05 against control group, ***denotes p < 0.001 against control group. ns, not significant against control group. CsA, cyclosporine A.
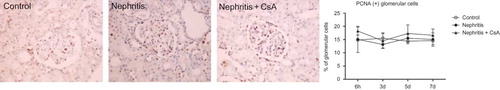
The expression of proliferating cell nuclear antigen (PCNA) in tubulointerstitium was significantly increased in the nephritis group during the experimental period compared to controls (, 14% in nephritis group vs. 3% in controls, p < 0.001). CsA treatment significantly decreased expression of PCNA in ATS injected rats at sixth hour (14% in nephritis group vs. 5% in nephritis + CsA group, p < 0.001) and 3rd day (14% in nephritis group vs. 4% in nephritis + CsA group, p < 0.001), but on following days PCNA expression was similar to ATS-treated rats. Glomerular expression of PCNA was similar (15–20%) among all groups ().
Figure 5. PCNA expression in tubulointerstitium of rat kidneys during the experimental period and histological appearances of kidneys in control (at sixth hour), nephritis (at sixth hour), and nephritis + CsA (at sixth hour) groups. Values are expressed as mean ± SEM. n = 6 at each time point for each group. Notes: ***Denotes p < 0.001 against control group. ns, not significant against control group. CsA, cyclosporine A.
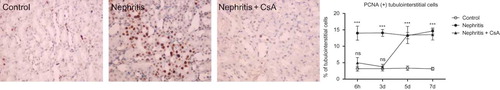
Figure 6. PCNA expression in glomeruli of rat kidneys during the experimental period and histological appearances of kidneys in control (at sixth hour), nephritis (at sixth hour), and nephritis + CsA (at sixth hour) groups. Values are expressed as mean ± SEM. n = 6 at each time point for each group. Note: CsA, cyclosporine A.
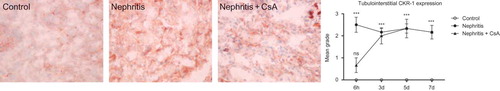
CXCR1 was expressed in neither glomeruli nor tubulointerstitium of control rats. ATS injection resulted in expression of CXCR1 in both glomeruli and tubulointerstitium, which started in early period after ATS injection and persisted during the experiments. CsA treatment precluded CXCR1 expression in both glomeruli and tubulointerstitium ( and , p < 0.001 and p < 0.001 respectively) 6 h after ATS injection, but had no effect on expression in the following days.
Figure 7. CXCR1 expression in glomeruli of rat kidneys during the experimental period and histological appearances of kidneys in control (grade 0, at sixth hour), nephritis (grade 4, at sixth hour), and nephritis + CsA (grade 0, at sixth hour) groups. Values are expressed as mean ± SEM. n = 6 at each time point for each group. Notes: *Denotes p < 0.05 against control group, ***denotes p < 0.001 against control group. ns, not significant against control group. CsA, cyclosporine A.
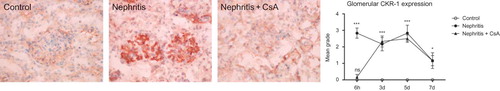
Figure 8. CXCR1 expression in tubulointerstitium of rat kidneys during the experimental period and histological appearances of kidneys in control (grade 0, at sixth hour), nephritis (grade 3, at sixth hour), and nephritis + CsA (grade 1, at sixth hour) groups. Values are expressed as mean ± SEM. n = 6 at each time point for each group. Notes: ***Denotes p < 0.001 against control group. ns, not significant against control group. CsA, cyclosporine A.
DISCUSSION
In ATS-induced mesangioproliferative glomerulonephritis model, we observed that CXCR1 was highly expressed in both glomeruli and tubulointerstitium starting on the first day of experimental protocol and this high level was maintained in tubulointerstitium until the end of the first week. In addition, CXCR1 expression correlated with interstitial inflammation and mesangial proliferation. These observations may suggest a role of CXCR1 on leukocyte recruitment to inflammation site in experimental mesangioproliferative glomerulonephritis.
Several groups found an accumulation of neutrophils within glomerulus and tubulointerstitium of human kidneys very similar to the general distribution of CXCR1 staining demonstrated in the present study.Citation17,18 The highest numbers of glomerular CXCR1-positive cells, which are consistent with polymorphonuclear leukocytes (PMNs) have been found to be present in biopsies of patients with membranoproliferative glomerulonephritis, followed by lupus nephritis and crescentic glomerulonephritis.Citation17 Expression of IL-8 and CXCR1 mRNA in glomeruli with crescentic glomerulonephritis and lupus nephritis has also been demonstrated.Citation17 A pathogenic role of CXCR1-positive PMNs can easily be appreciated as these cells are a rich source for proteases, reactive oxygen species, and cytokines that promote cellular activation and further recruitment of inflammatory cells. Infiltration of glomeruli and other renal compartments by inflammatory cells, which correlates with prognosis, is hallmark of various forms of glomerulonephritis.Citation19 In recent years, growing evidence regarding their roles in inflammation, chemokines and their receptors have been proposed to be regarded as attractive therapeutic targets for treatment of inflammatory renal diseases such as glomerulonephritis.Citation19,20 Indeed, in vivo treatment with an IL-8-blocking antibody reduced glomerular neutrophil accumulation and proteinuria in an acute immune complex glomerulonephritis in rabbits.Citation21 CXCR1, a receptor of IL-8, has been shown to be expressed on podocytes in vitro and in vivo during membranous glomerulonephritis,Citation22 as well as on neutrophils in membranoproliferative glomerulonephritis, lupus nephritis, and crescentic glomerulonephritis.Citation17 Polymorphisms in IL-8 and CXCR2 genes are associated with disease progression in childhood IgA nephropathy.Citation23 Our group previously demonstrated significantly increased urinary and serum IL-8 levels in the acute phase of acute poststreptococcal glomerulonephritis when compared to resolution phase and controls.Citation10 The current study and the previous clinical studies underline the importance of IL-8 and corresponding receptors in glomerular inflammation.
In the present study, CXCR1 expression in glomeruli started to decline at the end of first week, but increased glomerular inflammation and mesangial proliferation persisted. It seems that CXCR1 is responsible for initiation but not continuation of inflammation. The recruitment of CXCR1-positive PMNs could be an early event, and might promote downstream recruitment of macrophages and proliferation of other intrinsic renal cells both of which contribute to maintenance of cellular accumulation as observed in human membranoproliferative glomerulonephritis.Citation17
In the present study, we also showed that single-dose CsA treatment efficiently but transiently decreased glomerular and tubulointerstitial CXCR1 expression and corresponding inflammation and proteinuria. Whether or not repetitive administrations might yield stable effect remained undetermined. To the best of our knowledge, the effect of CsA treatment on CXCR1 expression has not been investigated previously, but Wakabayashi et al. showed that calcineurin inhibitors (CsA and FK-506) inhibit IL-8 expression in glioma cells induced by calcium but not TNF-α.Citation24 Although, the precise pathway is unknown, a calcium–calcineurin–NF-κB pathway leads to IL-8 expression in glioma cells. The early protective effect of CsA demonstrated in our study might be a result of decreased IL-8 expression and/or down-regulation of CXCR1 expression secondary to decreased IL-8 expression driven by CsA. On the other hand, Huber et al. demonstrated that stimulation of CXCR1 leads a concentration-dependent increase in cytosolic-free Ca+2 concentration in cultured podocytes and that stimulation of chemokine receptors leads to release of IL-8 in cultured podocytes, which in turn down-regulates the expression of CXCR1 on these cells via receptor internalization.Citation22 Since these two in vitro studies were performed in cultured glioma cells and podocytes, further in vivo studies are needed to elucidate the exact mechanisms.
In summary, this is the first study that demonstrates expression of CXCR1 in both glomerular and tubulointerstitial renal compartments, and its relation with proteinuria and also effect of single-dose CsA on these parameters in ATS-induced mesangioproliferative glomerulonephritis. We showed that CXCR1 might contribute to inflammation in both glomeruli and tubulointerstitium in this model. Single-dose CsA exerts a temporary protective effect that is parallel to down-regulation of CXCR1 expression. Long-term effects of repetitive administrations as well as actions of CsA on the chemokine receptors are subjects of further researches. The pathogenic role of chemokines and their receptors is still incompletely understood, but these systems may represent novel therapeutic targets in humans.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
This study was supported by The Scientific Research and Development Office of the Hacettepe University (0102101023).
REFERENCES
- Stahl R, Thaiss F, Oberle G, The platelet activating factor receptor antagonist WEB 2170 improves glomerular hemodynamics and morphology in a proliferative model of mesangial cell injury. J Am Soc Nephrol. 1991;2:37–44.
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568.
- Baggiolini M. Chemokines in pathology and medicine. J Int Med. 2001;250:91–104.
- Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135.
- Segerer S, Nelson PJ, Schlondorf D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–176.
- Segerer S, Alpers CE. Chemokines and chemokine receptors in renal pathology. Curr Opin Nephrol Hypertens. 2003;12:243–249.
- Murphy PM, Baggiolini M, Charo IF, International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176.
- Yoshimura T, Matsushima K, Tanaka S, Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237.
- Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–761.
- Besbas N, Ozaltin F, Catal F, Ozen S, Topaloglu R, Bakkaloglu A. Monocyte chemoattractant protein-1 and interleukin-8 levels in children with acute poststreptococcal glomerulonephritis. Pediatr Nephrol. 2004;19:864–868.
- Brown Z, Strieter RM, Chensue SW, Cytokine-activated human mesangial cells generate the neutrophil chemoattractant, interleukin 8. Kidney Int. 1991;40:86–90.
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–543.
- Razzaque MS, Taguchi T. Collagen-binding heat shock protein (HSP) 47 expression in anti-thymocyte serum (ATS)-induced glomerulonephritis. J Pathol. 1997;183: 24–29.
- Hayashi K, Osada S, Shofuda K, Horikoshi S, Shirato I, Tomino Y. Enhanced expression of membrane type-1 matrix metalloproteinase in mesangial proliferative glomerulonephritis. J Am Soc Nephrol. 1998;9:2262–2271.
- Bagchus WM, Hoedemaeker PJ, Rozing J, Bakker WW. Glomerulonephritis induced by monoclonal anti-Thy 1.1 antibodies. A sequential histological and ultrastructural study in the rat. Lab Invest. 1986;55:680–687.
- Otani H, Mune M, Yukawa S, Smith D, Meydani M, Blumberg J. Vitamin E treatment of experimental glomerular disease in rats. Kidney Int. 1999;56:S66–S69.
- Segerer S, Henger A, Schmid H, Expression of the chemokine receptor CXCR1 in human glomerular diseases. Kidney Int. 2006;69:1765–1773.
- Cockwell P, Brooks CJ, Adu D, Savage COS. Interleukin-8: a pathogenetic role in antineutrophil cytoplasmic autoantibody-associated glomerulonephritis. Kidney Int. 1999;55:852–863.
- Yu HT. Progression of chronic renal failure. Arch Intern Med. 2003;163:1417–1429.
- Anders HJ, Sayyed SA, Vielhauer V. Questions about chemokine and chemokine receptor antagonism in renal inflammation. Nephron Exp Nephrol. 2009;114:e33–e38.
- Wada T, Tomosugi N, Naito T, Prevention of proteinuria by the administration of anti-interleukin 8 antibody in experimental acute immune complex-induced glomerulonephritis. J Exp Med. 1994;180:1135–1140.
- Huber TB, Reinhardt HC, Exner M, Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol. 2002;168:6244–6252.
- Suh JS, Hahn WH, Cho BS. Polymorphisms of CXCL8 and its receptor CXCR2 contribute to the development and progression of childhood IgA nephropathy. J Interferone Cytokine Res. 2011;31:309–315.
- Wakabayashi K, Kambe F, Cao X, Inhibitory effects of cyclosporin A on calcium mobilization-dependent interleukin-8 expression and invasive potential of human glioblastoma U251MG cells. Oncogene. 2004;23:6924–6932.