Abstract
Background: Elevated serum uric acid has been associated with a variety of cardiovascular disease and with inflammation, but these have been little explored in chronic kidney disease (CKD). Elevated uric acid levels are common in CKD patients and could be involved in inflammatory milieu; our aim was to analyze the association between uric acid and inflammatory markers in hemodialysis (HD) patients. Design: This was a cross-sectional study. Setting: This study was conducted from private clinic, Rio de Janeiro, Brazil. Patients: This study included 50 HD patients and 21 healthy subjects. Methods and procedures: This study included 50 HD patients [62% men, 54.3 ± 12.6 years, 57.5 ± 50.1 months on dialysis, and body mass index (BMI), 24.4 ± 4.1 kg/m2] and 21 healthy individuals (45% men, 50.7 ± 15.7 years and BMI, 25.5 ± 4 kg/m2). Uric acid was measured using uricase-PAP method; inflammatory [tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP)] and atherosclerosis markers [intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1)] were measured by a multiplexed assay. Results: Patients presented high levels of TNF-α, IL-6, CRP, VCAM-1, ICAM-1 (5.5 ± 2.1 pg/mL, 4.1 ± 1.6 pg/mL, 0.32 ± 0.30 mg/mL, 48.5 ± 8.5 ng/mL, 20.5 ± 15.9 ng/mL, respectively), compared with healthy individuals (2.4 ± 1.1 pg/mL, 2.7 ± 0.4 pg/mL, 0.11 ± 0.12 mg/mL, 23.8 ± 5.5 ng/mL, 7.2 ± 1.2 ng/mL, respectively) ( p < 0.04). Uric acid levels were also higher in HD patients (5.4 ± 1.3 mg/dL) than in healthy individuals (3.9 ± 0.9 mg/dL) ( p < 0.02). There was a positive correlation between uric acid and inflammatory markers, IL-6 (r = 0.30, p = 0.01), CRP (r = 0.37, p = 0.003), TNF-α (r = 0.40, p = 0.001), ICAM-1 (r = 0.53, p = 0.0001), and VCAM-1 (r = 0.45, p = 0.0001). Conclusion: These original data suggest that uric acid may have a role in inflammation and atherosclerosis in HD patients. However, further prospective studies involving intervention trials should be conducted in order to search for actual causality relationship between these markers.
INTRODUCTION
Uric acid is an end product of purine metabolism in human and higher primates produced via the action of xanthine oxidase, an enzyme that is implicated in oxidative processes.Citation1,2
Uric acid has been clearly associated with oxidative stress and inflammation in several pathological conditionsCitation1,3–6 and it has emerged as a simple and independent marker of morbidity and mortality in a variety of cardiovascular disease states.Citation7–10
In studies including only few patients with chronic heart failure, uric acid was associated positively with tumor necrosis factor-alpha (TNF-α)Citation11,12 and interleukin-6 (IL-6).Citation11 In two population-based studies including 566Citation13 and 1703Citation14 healthy people, uric acid was associated positively with C-reactive protein (CRP). Ruggiero et al.Citation15 observed that uric acid was positively associated with IL-6, TNF-α, and CRP in elderly people and in 2007, these authors observed that uric acid predicted CRP increase during follow-up.Citation16 In addition, Bo et al.Citation17 observed an inverse association between uric acid and adiponectin and direct association with E-selectin in a sample of 100 men from a population-based cohort. Recently, Lyngdoh et al.Citation18 showed that uric acid was associated positively with IL-6, CRP, and TNF-α and negatively with interleukin-1 beta (IL-1β) in a cross-sectional population-based study. Baldwin et al.Citation19 demonstrated in vitro that uric acid induced the production (mRNA and secreted protein) of monocyte chemotactic protein-1 (MCP-1) and caused a decrease in the production of adiponectin.
These findings suggest that uric acid is an actual endothelium-injuring factor. It is therefore justified to consider uric acid an important risk factor for vascular disease and atherosclerosis.
Few studies have investigated the relationship between uric acid and systemic inflammation in chronic kidney disease (CKD) patients; however, they have showed a relationship between uric acid and CRP, intercellular adhesion molecule-1 (ICAM-1) levels and also with all-cause mortality.Citation20–22
Nevertheless, serum uric acids increase in patients with reduced renal function due to impaired renal excretion and levels of 7.0 mg/dL or higher are present in ∼50% of subjects on dialysis.Citation23 Due to paucity of data and based on the hypothesis that uric acid is involved in inflammation, our aim was to investigate whether serum uric acid levels are correlated to inflammatory markers in patients undergoing hemodialysis (HD).
METHODS
Subjects
A descriptive cross-sectional study was conducted in a group of 50 HD patients (62% men, 54.3 ± 12.6 years, 57.5 ± 50.1 months on dialysis, and BMI, 24.4 ± 4.1 kg/m2) from a private clinic in Rio de Janeiro, Brazil and compared with 21 healthy individuals, without any disease, who were not on any medication (45% men, 50.7 ± 15.7 years, BMI 25.5 ± 4 kg/m2). Inclusion criteria were age older than 18 years, patients who had been on maintenance dialysis for at least 6 months, and without use of lipid-lowering medicines and uric acid-lowering drugs. Patients with cancer, AIDS, autoimmune diseases, and the previous use of a catheter for dialysis access were excluded. The control group studied was healthy individuals, without any disease, who were not on any medication. The dialysis duration was a 3–4.5-h session three times per week, blood flow greater than 250 mL/min, dialysate flow 500 mL/min, and bicarbonate buffer. Renal failure etiologies included systemic arterial hypertension (n = 23), diabetes (n = 11), chronic glomerulonephritis (n = 6), polycystic kidney disease (n = 3), and unknown (n = 7). The average arterial blood pressure of patients was 122.2 × 73.7 mmHg. Regarding anti-hypertensive treatments in the HD patients, 30% of the patients were receiving medications including calcium channel blockers (n = 5), ACE-inhibitors (n = 6), or in combination (n = 3). The study protocol was approved by Ethics Committee of Clementino Fraga Filho University Hospital (HUCCF-UFRJ-066/08).
Nutritional Assessment
The following anthropometric parameters were measured: body weight, height, and waist circumference. Body mass index (BMI) was calculated from the equation: BMI = weight (kg)/height2 (m).Citation24 Measurements were made after the dialysis session by a trained staff member.
Biochemical Analyses
Blood samples were obtained from the arterial line of the hemodialysis immediately before the HD session, after the subjects had fasted overnight. The serum was immediately frozen at –80°C until analyzed. To measure IL-6, CRP, TNF-α, PAI-1, and MCP-1, we utilized the base kit—Human Obesity MultiAnalyte Profiling Base Kit (LOB000) and the beat sets: TNF-α (catalog number LUH2010/microparticle region 77), IL-6 (catalog number LUH206/microparticle region 32), MCP-1 (catalog number LUH279/microparticle region 78), PAI-1 (catalog number LOB1786/microparticle region 10), CRP (catalog number LOB1707/microparticle region 8). To measure VCAM-1 and ICAM-1, we utilized the kit base Human Adhesion Molecule MultiAnalyte Profiling Base Kit (LAD000) and the beat sets: VCAM-1 (catalog number LAD 809/microparticle region 20) and ICAM-1 (catalog number LAD720/microparticle region 18). All particles were measured with a multiplex assay kit manufactured by R&D Systems (Minneapolis, MN, USA) in a Luminex® 100/200™ System (Austin, TX, USA), following manufacturer’s instructions. Serum uric acid was measured by standard analytical methods (Clauss technique and uricase enzymatic test, respectively; normal range of uric acid levels: 3.4–7 mg/dL for men and 2.4–6 mg/dL for women).
Statistical Analysis
Data are presented as mean ± SD or median and range when appropriate. A p-value <0.05 were considered statistically significant. The statistical significance of differences was analyzed by t-test one-way analysis or the non-parametric Mann–Whitney test, as appropriate. The Spearman or Pearson correlation coefficient was used to determine the relationships between clinical parameters. The multiple regression analysis was used to determine which factor mainly contributes to plasma levels of proinflammatory cytokines and atherosclerosis markers. Statistical analyses were performed using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA).
RESULTS
The demographic, clinical and laboratory characteristics of the patients and healthy individuals are listed in . The patients presented significantly high levels of TNF-α, IL-6, CRP, VCAM-1, and ICAM-1 compared with healthy individuals. Uric acid levels were also higher in HD patients (5.4 ± 1.3 mg/dL) than in healthy individuals (3.9 ± 0.9 mg/dL) (p < 0.02) and there was no difference according to gender. The difference of PAI-1 and MCP-1 between HD and healthy individuals was not statistically significant. The parameters analyzed were not different between diabetic and nondiabetic patients.
Table 1. General and biochemical characteristics of the HD patients and healthy individuals.
Table 2. Multivariate regression analysis.
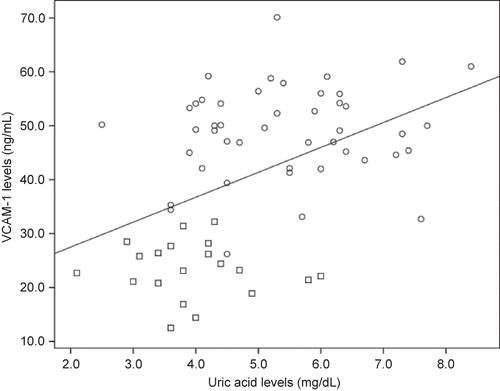
There was a positive correlation between serum uric acid and IL-6 (r = 0.30, p = 0.01, n = 66), CRP (r = 0.37, p = 0.003, n = 61), TNF-α (r = 0.40, p = 0.001, n = 64) (), ICAM-1 (r = 0.53, p = 0.0001, n = 66) (), and VCAM-1 levels () (r = 0.45, p = 0.0001, n = 66). Likewise, there was correlation between VCAM-1 and TNF-α (r = 0.59, p = 0.0001, n = 65), and between ICAM-1 and CRP (r = 0.52, p = 0.0001, n = 60) and IL-6 (r = 0.58, p = 0.0001, n = 67).
The independent factors affected by uric acid levels in multivariate regression analysis were IL-6, TNF-α, CRP, ICAM-1, and VCAM-1 ().
DISCUSSION
The most important finding of the present study was the correlation between uric acid and IL-6, CRP, TNF-α, ICAM-1, and VCAM-1 levels in HD patients. These results support the hypothesis that uric acid is involved in inflammation by triggering the release of inflammatory cytokines.
A great number of studies in general populations have identified that elevated uric acid has been clearly associated with oxidative stress and inflammation in several pathological conditions and it is associated with morbidity and mortality in a variety of cardiovascular disease states.Citation1,3–6,8–10
The mechanisms by which uric acid induce inflammation seems to involve complex pathways that are able to induce oxidative stress and endothelial dysfunction. Through the activation of mitogen-activated protein kinases and extracellular signal-regulated kinase 1 and 2 (Erk 1/2) phosphorylation, uric acid increases the expression and the activity of cyclo-oxygenase-2.Citation25 Uric acid is known to act as a host-derived danger-associated molecular pattern by directly activating the Nod-like receptor (NLR), NALP3 (NLRP3), and activation of NALP3/NLRP3 induce IL-1β release, a potent proinflammatory cytokine, which signals via its receptor (IL-1R) on parenchymal cells, and along with other chemotactic factors, it serves as a beacon to attract neutrophils and other immune cells, thereby eliciting an inflammatory response to tissue injury.Citation26
Clinical and epidemiological studies have unequivocally confirmed the association between high levels of uric acid and cardiovascular disease.Citation6,10,27–32
Hyperuricemia is common in CKD patients, including patients on HD and peritoneal dialysis patients.Citation33 Uric acid is a small water-soluble compound that is removed by hemodialysis from plasma in a similar way as urea, but its removal from the intracellular compartment is by far not as efficient as that of urea.Citation34 Shahbazian et al.Citation23 assessed the plasma concentration uric acid before and after HD and observed that uric acid levels in HD patients are higher than normal before hemodialysis, and they decrease significantly after hemodialysis; however, both of them may be still higher than normal values.
Few studies have investigated the relationship between uric acid and inflammation in CKD patients. In line with our results, Caravaca and colleaguesCitation21 observed a relationship between uric acid and CRP levels that remained statistically significant after adjustment for age, sex, comorbid index, obesity, residual renal function, diuretic, and allopurinol treatment and even after the inclusion of HOMA-IR and fasting insulin levels as independent variables in patients without diabetes, concluding that uric acid levels are related with CRP levels in CKD patients. Suliman et al.Citation22 demonstrated that serum uric acid levels positively correlated with CRP and ICAM-1 in CKD patients starting dialysis.
Tang et al.Citation35 observed an independent correlation between uric acid and flow-mediated dilatation, and a higher uric acid level was related to worse endothelial function which may contribute to hypertension and cardiovascular morbidity in continuous ambulatory peritoneal dialysis patients. In fact, serum uric acid levels showed a J-shaped association with all-cause mortality in CKD patients.Citation20,22
Besides the traditional risk factors, Mutluay et al.Citation36 showed that uric acid is one of the factors that may have a predictive role in recognizing premature atherosclerosis in chronic kidney disease patients. It has been observed by Kanbay and associatesCitation37 that uric acid was independently determinant of severity of coronary artery disease.
Therefore, the above studies taken together compose substantial evidence that links uric acid with diverse cardiovascular pathologies, and our findings support the theory that high uric acid may contribute to the atherosclerotic process by stimulating the release of inflammatory cytokines.
Figure 1. Correlation between uric acid levels and TNF-α.Notes: Square denotes healthy individuals; Cirlcle denotes HD patients. r = 0.40, p = 0.001; n = 64.
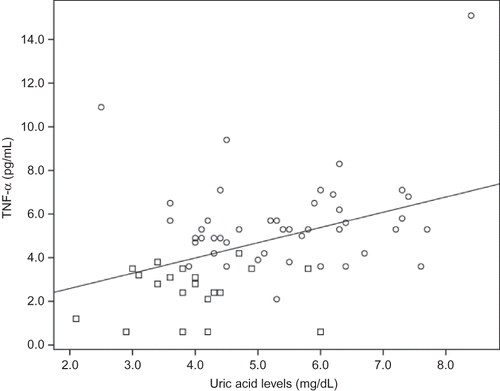
Figure 2. Correlation between uric acid levels and ICAM-1.Notes: Square denotes healthy individuals; Cirlcle denotes HD patients. r = 0.53, p = 0.0001; n = 66.
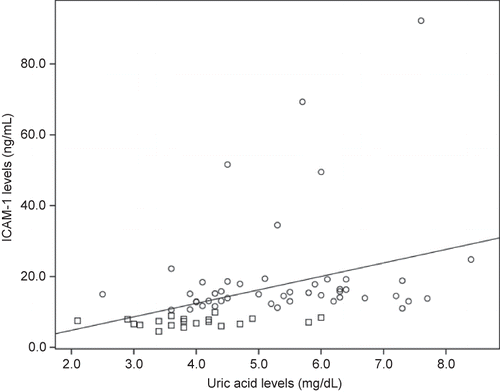
Figure 3. Correlation between uric acid levels and VCAM-1.Notes: Square denotes healthy individuals; Cirlcle denotes HD patients. r = 0.45, p = 0.0001; n = 66.
It is important to note that, in our study few patients had high levels of uric acid and despite of it, uric acid was correlated with inflammatory markers. These data may indicate the need to reduce the reference values for uric acid to prevent cardiovascular complications in CKD patients.
Uric acid elevation seems to be an excellent candidate for primacy among cardiorenal risk factors. However, of course, to establish causality, and not mere co-incidence, we need interventional outcome studies revealing an improvement in renal, cardiac, or other outcomes after uric acid management. It is conceivable that since uric acid levels are associated with systemic inflammation, it could lead to endothelial dysfunction and increase the susceptibility to atherosclerosis in HD patients.
The most important limitation of our study is our inability to infer causality from the observed associations. This limitation is inherent in cross-sectional and observational studies and our study was also limited in that the number of patients included was relatively small.
In conclusion, these original data suggest that uric acid may have a role in inflammation and atherosclerosis in hemodialysis patients. However, further prospective studies involving intervention trials should be conducted in order to search for actual causality relationship between these markers.
PRACTICAL APPLICATION
The present study is very important because we can provide recommendations for nephrology healthcare about uric acid, since patients with low levels of uric acid had higher inflammatory markers levels. These data may indicate the need to reduce the reference values for uric acid to prevent cardiovascular complications in CKD patients.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article apart from those disclosed.
ACKNOWLEDGMENT
This study was supported by Faperj (Fundação de Amparo à Pesquisa do estado do Rio de Janeiro) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
REFERENCES
- Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17:409–414.
- Shi Y, Mucsi AD, Ng G. Monosodium urate crystals in inflammation and immunity. Immunol Rev. 2010;233:203–217.
- Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25:39–42.
- Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151.
- Corry DB, Tuck ML. Uric acid and the vasculature. Curr Hypertens Rep. 2006;8:116–119.
- Letsas KP, Korantzopoulos P, Kardaras F, . Uric acid elevation in atrial fibrillation. Hellenic J Cardiol. 2010;51:209–213.
- Dawson J, Walters M. Uric acid and xanthine oxidase: future therapeutic targets in the prevention of cardiovascular disease? Br J Clin Pharmacol. 2006;62:633–644.
- Alderman MH. Podagra, uric acid, and cardiovascular disease. Circulation. 2007;116:880–883.
- So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120(6):1791–1799.
- Basar N, Sen N, Ozcan F, . Elevated serum uric acid predicts angiographic impaired reperfusion and 1-year mortality in ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention. J Investig Med. 2011;56(9):931–937.
- Leyva F, Anker SD, Godsland IF, . Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J. 1998;19:1814–1822.
- Olexa P, Olexova M, Gonsorcik J, . Uric acid—a marker for systemic inflammatory response in patients with congestive heart failure? Wien Klin Wochenschr. 2002;112:211–215.
- Saito M, Ishimitsu T, Minami J, . Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;197:73–79.
- Frohlich M, Imhof A, Berg G, . Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839.
- Ruggiero C, Cherubini A, Ble A, . Uric acid and inflammatory markers. Eur Heart J. 2006;27:1174–1181.
- Ruggiero C, Cherubini A, Miller E, . Usefulness of uric acid to predict changes in C-reactive protein and interleukin-6 in 3-year period in Italians aged 21 to 98 years. Am J Cardiol. 2007;100:115–121.
- Bo S, Gambino R, Durazzo M, . Associations between serum uric acid and adipokines, markers of inflammation, and endothelial dysfunction. J Endocrinol Invest. 2008;31(6):499–504.
- Lyngdoh T, Marques-Vidal P, Paccaud F, . Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011; 6(5):e19901.
- Baldwin W, McRae S, Marek G, . Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60(4):1258–1269.
- Hsu SP, Pai MF, Peng YS, . Serum uric acid levels show a ‘J-shaped’ association with all-cause mortality in hemodialysis patients. Nephrol Dial Transplant. 2004;19:457–462.
- Caravaca F, Martín MV, Barroso S, . Serum uric acid and C-reactive protein levels in patients with chronic kidney disease. Nefrologia. 2005;25(6):645–654.
- Suliman ME, Johnson RJ, Garcia-Lopez E, . J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis. 2006;48:761–771.
- Shahbazian H, Moghadam AZ, Ehsanpour A, Khazaali M. Changes in plasma concentrations of hypoxanthine and uric acid before and after hemodialysis. IJKD. 2009;3:151–155.
- Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–343.
- Feig DI, Mazzali M, Kang DH, . Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol. 2006;17:69–73.
- Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30:628–631.
- Mazzali M, Kanellis J, Han L, . Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:991–997.
- Kang DH, Park SK, Lee IK, . Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562.
- Hozawa A, Folsom AR, Ibrahim H, Javier Nieto F, Rosamond WD, Shahar E. Serum uric acid and risk of ischemic stroke: the ARIC Study. Atherosclerosis. 2006;187:401–407.
- Rodrigues TC, Maahs DM, Johnson RJ, . Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care. 2010;33(11):2471–2473.
- Heo SH, Lee SH. High levels of serum uric acid are associated with silent brain infarction. J Neurol Sci. 2010;297:6–10.
- Silbernagel G, Hoffmann MM, Grammer TB, Boehm BO, März W. Uric acid is predictive of cardiovascular mortality and sudden cardiac death in subjects referred for coronary angiography. Nutr Metab Cardiovasc Dis. 2013;23(1):46–52.
- Chen Y, Ding X, Teng J, . Serum uric acid is inversely related to acute ischemic stroke morbidity in hemodialysis patients. Am J Nephrol. 2011;33(2):97–104.
- Langsdorf LJ, Zydney AL. Effect of uremia on the membrane transport characteristics of red blood cells. Blood. 1993;81:820–827.
- Tang Z, Cheng LT, Li HY, Wang T. Serum uric acid and endothelial dysfunction in continuous ambulatory peritoneal dialysis patient. Am J Nephrol. 2009;29(5):368–373.
- Mutluay R, Konca C, Erten Y, . Predictive markers of asymptomatic atherosclerosis in end-stage renal disease patients. Ren Fail. 2010;32(4):448–454.
- Kanbay M, Yilmaz MI, Sonmez A, . Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol. 2011;33(4):298–304.