Abstract
Introduction: Neurological complications secondary to the uremic state, contribute largely to the morbidity and mortality in patients with renal failure. The prevalence of peripheral neuropathy remains high in advanced renal dysfunction. Materials and methods: The present cross-sectional study was conducted on 100 adult patients of chronic kidney disease between 18 and 75 years of age with serum creatinine greater than 2 mg/dL. Apart from routine examination and baseline investigations, detailed history was elicited pertaining to patients’ neurological symptoms, and scored according to the Neurological Symptom Score. Motor nerve conduction velocity was measured from right median, ulnar, peroneal, and tibial nerves. Results: It was observed that neurological symptoms increased steadily with raise in serum creatinine. The mean nerve conduction velocities (NCVs) of right median nerve, ulnar nerve, peroneal nerve, and tibial nerve were 51.34 ± 6.07, 53.04 ± 5.91, 44.72 ± 6.14, and 44.20 ± 5.17, respectively. The NCVs of all the tested nerves decreased significantly with increase in serum creatinine levels (p < 0.01): 70% of the patients had uremic polyneuropathy; 6% had asymptomatic neuropathy, 51% had symptomatic non-disabling neuropathy, while disabling neuropathy was seen in 13% of the patients. Conclusion: Our data suggests that NCV testing when complimented with meticulous neurological assessment can provide invaluable input. These tests apart from helping us detect neuropathy in advanced renal dysfunction; can also detect the disease in largely asymptomatic patients which avoids the necessity to order for detailed neurophysiological investigation
Introduction
Chronic Kidney Disease (CKD) is a major public health problem in developed and developing countries alike leading to decreased quality of life across the globe. It is a well-known fact that patients of CKD are at increased risk for mortality as well as morbidity due to the myriad complications associated with this disease entity. Neurological complications, secondary to the uremic state, contribute largely to the morbidity and mortality in patients with renal failure. Despite continuous therapeutic advances, many neurological complications of uremia, like uremic encephalopathy, atherosclerosis, neuropathy, and myopathy fail to respond completely to these treatment modalities.
Although access to comprehensive dialysis programs and transplant facilities has become nothing short a routine protocol worldwide, the prevalence of neurological symptoms remains high in advanced renal dysfunction. Recent studies of neuropathy in the end stage renal disease (ESRD) have demonstrated that 70–100% of patients on dialysis experience neuropathic symptoms, despite attaining current targets of dialysis adequacy.Citation1–3 As almost all these patients will invariably develop neuromuscular disease, understanding the pathophysiological processes underlying neuropathic symptoms in ESRD patients is of highest priority for nephrologists dealing with this issue on routine basis.
The most common neurological complication of CKD is peripheral neuropathy. The development of neuropathy has been widely attributed to the degree of renal impairment, with clinically significant neuropathy said to occur after the glomerular filtration rate drops to less than 12 mL/minute.Citation4 Recent advances in modalities of renal replacement have failed miserably to bring about any substantial reduction in the prevalence of neuropathy in this population. Although the prevalence of severe neuropathy may appear to have decreased to a certain extent, a significant cohort of ESRD patients still report symptoms which are considered functionally disabling, and even patients who meet accepted guidelines for dialysis adequacy may complain of neuropathic symptoms.Citation1,Citation5 Renal transplantation remains the only known cure for uremic neuropathy, with clinical improvement in sensory and, to a lesser extent, motor function occurring within a few days of transplantation.Citation6,Citation7
Uremic neuropathy characteristically presents as a slowly progressive neuropathy which is symmetrical and length-dependent, initially affecting the distal limbs, and gradually progressing more proximally.Citation8,Citation9 The earliest symptoms usually reflect sensory dysfunction, resulting in paraesthesia, pain, and numbness mainly confined to the lower limbs, characteristically exhibiting a pattern of distal “stocking” sensory loss. With more severe disease, motor involvement develops, characterized by weakness, and muscle atrophy, again most prominent distally. Involvement of proximal regions of the lower limbs and upper limb develops in advanced disease.
Peripheral neuropathy in CKD has been extensively studies since this condition was first described in ESRD patients on hemodialysis. Despite its significant contribution to morbidity in CKD patients, clinicians in India tend to repetitively ignore the prevalence of this entity and the discomfort it causes to the patients, channelizing their treatment protocol toward managing the more severe and life threatening complications of CKD. No significant contribution has been made in detection or management of uremic neuropathy in Indian literature. Our hospital, a tertiary centre caterings to the needs of entire state of Haryana, provides a significant platform to study the prevalence of this disease. We hereby have planned this study to assess the spectrum of peripheral neuropathy in patients of CKD, and correlate it with the severity of renal dysfunction.
Materials and methods
The present study was conducted on 100 adult patients of chronic kidney disease between 18 and 75 years of age, on regular follow-up of Kidney and Dialysis clinic of Pt. B. D. Sharma PGIMS, Rohtak, with persistently elevated serum creatinine greater than 2 mg/dL. A pre-informed written consent for enrolment in the study was obtained. Patients on hemodialysis and those who had received renal transplant were excluded from the study. Also excluded were patients with diabetes mellitus, vitamin B12 deficiency, collagen vascular disorders, hypothyroidism, pre-existing peripheral neuropathy, malignancy, and patients on drugs known to cause peripheral neuropathy.
They were subjected to detailed history, general-physical examination, and neurological examination. History regarding any previous/concomitant illness intake of drugs, prescription as well as recreational, were elicited and recorded when deemed relevant. Patients’ neurologic symptoms were scored as per Neurological Symptom Score (NSS). Their routine renal and other biochemical investigations including blood urea (mg/dL), serum creatinine (mg/dL), serum corrected calcium levels (mg/dL), serum phosphorous levels (mg/dL), calcium phosphate product, serum protein (g/dL), iPTH levels (pg/mL), vitamin B12 levels(pg/mL), serum sodium (meq/L), serum potassium (meq/L), blood sugar were carried out as per the standard methods used in the Department of Biochemistry, PGIMS, Rohtak, while eGFR was estimated using MDRD equation. Each patient was also subjected to nerve conduction studies (NCS) to determine motor nerve conduction velocities (NCVs) of median, ulnar, peroneal, and tibial nerves unilaterally (right). Patients continued receiving anti-hypertensives, diuretics, iron, and calcium supplements as per their renal and biochemical profiles.
Detailed history was elicited pertaining to patients’ neurological symptoms, and scored according to the Neurological Symptom Score developed by Dyck. Each symptom was given a score of one and Total Neuropathic Symptom Score was calculated (T-NSS). Maximum possible T-NSS was 17 ().Citation10,Citation11
Table 1. Neurological symptom score.
An RMS Aleron 401 model electromyography was used for determining the nerve conduction velocities. For motor nerve conduction study on the median nerve, a 10 mm silver disc was placed on the skin over abductor pollicis brevis as recording electrode and reference electrode was placed 3 cm distal at first metacarpophalangeal joint, while a ground electrode was placed over the dorsum of the hand. The nerve was supra maximally stimulated first at the wrist (3 cm proximal to the distal wrist crease), followed by the elbow (near the volar crease of the brachial pulse). Similarly for ulnar nerve, motor nerve conduction velocity was measured by recording from abductor digiti minimi and stimulating the ulnar nerve at wrist (3 cm proximal to the distal crease) and elbow (3–4 cm distal to medial epicondyle); for peroneal nerve, surface recordings were obtained from extensor digitorum brevis and stimulation given at ankle and 2 cm distal to fibular neck; for tibial nerve, recording electrode was placed on abductor hallucis and stimulation given behind and proximal to medial malleolus and in the popliteal fossa along the flexor crease of knee. The time latency was measured from the point of stimulus to the onset of muscle response and expressed in milliseconds. Motor nerve conduction velocity was calculated by measuring the distance in millimeters between two points of stimulation (D), which was then divided by the latency difference in milliseconds.
Conduction velocity (NCV) = [Distance/Proximal latency – Distal latency] ms.
Peripheral neuropathy was considered to be present when patients’ detailed history, neurological examination and nerve conduction studies were consistent with the following criteria.
≥2 Abnormal NCV
≥1 Abnormal NCV + ≥1 NSS/Abnormality in neurological examination
≥2 NSS/Abnormality in neurological examination
The severity of neuropathy in the present study was staged using a modified form of a previously devised system, disability was defined as a curtailment in recreational activities, absence of feeling in the hand or inability to walk independently ().Citation10,Citation11
Table 2. Severity of neuropathy in the present study was staged using a modified form of a previously devised system, and disability was defined as a curtailment in recreational activities, absence of feeling in the hand or inability to walk independently.
After calculating the prevalence of peripheral neuropathy, the study population were divided into three groups which were defined as: Group A, comprising of patients of CKD with serum creatinine values between 2 and 3.4 mg/dL; Group B, comprising of patients with serum creatinine values between 3.5 and 4.9 mg/dL; Group C, comprising of patients with serum creatinine values ≥5 mg/dL. The prevalence of peripheral neuropathy was estimated in each of these and relation of motor nerve conduction velocity with severity of renal function in these patients was assessed.
Statistical analysis
At the end of the study, the data was collected and tabulated using Microsoft Excel 2010 version. Data was analyzed using SPSS version 20. All the continuous variables were expressed as mean ± 2 SD. Prevalence of peripheral neuropathy was expressed as percentage. Fischer’s Exact Test was applied to compare nominal data between the groups and p < 0.05 was considered significant. The relation of nerve conduction velocity with severity of renal function was assessed by the Pearson correlation coefficient; p < 0.05 was considered significant.
Observations
Among 100 patients included in the study, 68% were males and 32% were females respectively. Age of the patients varied from a minimum of 19 years to a maximum of 74 years. Mean age was 48.66 ± 13.07 and majority of the patients included in the study were above 30 years of age. Chronic glomerulonephritis was the leading cause of CKD in our study group affecting 38% of the patients, closely followed by hypertensive nephropathy (27%). Chronic interstitial nephritis (17%) and obstructive uropathy (12%) were the other common causes. Mean blood urea (mg/dL) was 106.03 ± 32.75; serum creatinine (mg/dL) was 3.81 ± 1.35; iPTH level (pg/mL) was 212.04 ± 178.73 and eGFR (mL/min) was 19.31 ± 8.10.
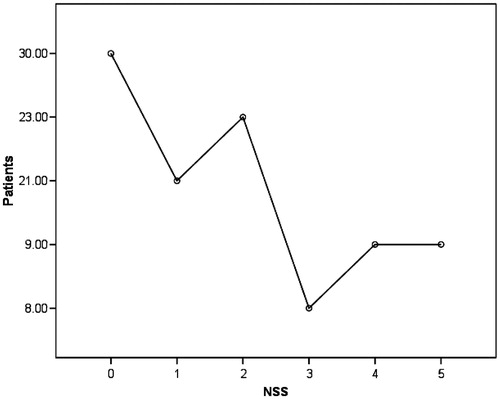
Neurological symptoms were assessed by Neurological Symptom Scoring (NSS). Majority of the patients had at least one neurological symptom (70%), as compared against the minority (30%) who were asymptomatic. A maximum of five neurological symptoms were elicited in 9% of the patients (). The most common symptom was paraesthesia/dysesthesia seen in 63% of the patients mostly confined to lower limbs, or involving both upper limbs and lower limbs. This was followed by complaints of weakness of hands/Limbs, in 35% of the patients. Patients also complained of unsteadiness in walking, postural fainting, and impotence. Moreover; it was observed that neurological symptoms as assessed by NSS increased steadily with raise in serum creatinine levels of patients.
Figure 1. Patients neurological symptoms as assessed by NSS. 70% of the patients had at least one neurological symptom. It was observed that neurological symptoms as assessed by NSS increased steadily with raise in serum creatinine levels of patients.
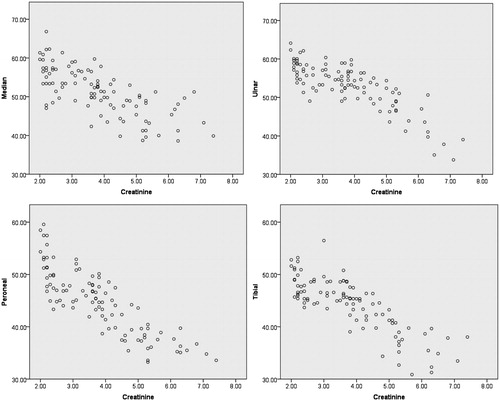
Uremic polyneuropathy was observed in 70% of the patients in our study. Meticulous neurological examination coupled with nerve conduction studies revealed a mixed sensory motor polyneuropathy, which is characteristic of uremic polyneuropathy. Among patients with neuropathy, 6% had asymptomatic neuropathy with ≥2 abnormal NCV and T-NSS of 0 (Stage 1). Symptomatic neuropathy; albeit non-disabling, was observed in 51% of the patients (Stage 2), while disabling neuropathy was seen in 13% of the patients (Stage 3) ().
Patients were assigned to different groups; each group defined by serum creatinine levels. Patients with serum creatinine values between 2 and 3.4 were assigned to Group A, and with serum creatinine values between 3.5–4.9 and ≥5 were assigned to Groups B and C respectively. Among 40 patients in Group A, 14 suffered from peripheral neuropathy (35%). Similarly among 37 patients in Group B, 33 were diagnosed to have peripheral neuropathy (89.19%). 100% prevalence was observed in Group C with all of the 23 patients affected by uremic polyneuropathy. This observation was extremely significant (<0.0001) between Groups A and B, and Groups A and C, respectively ().
Table 3. Patients with serum creatinine values between 2 and 3.4 were assigned to Group A, and with serum creatinine values between 3.5–4.9 and ≥5 were assigned to Groups B and C, respectively.
The mean NCVs of right median nerve, ulnar nerve, peroneal nerve, and tibial nerve were 51.34 ± 6.07, 53.04 ±5.91, 44.72 ± 6.14, and 44.20 ± 5.17, respectively. The nerve conduction velocities of all the tested nerves (median, ulnar, peroneal, and tibial) decreased with increase in serum creatinine levels. When NCVs of all the four nerves waere plotted against serum creatinine values and analyzed using the Pearson correlation, it showed a significant correlation (p < 0.01), suggesting that peripheral neuropathy worsened with rise in serum creatinine levels (). Furthermore, when NCV of each nerve was plotted against T-NSS, it revealed a steady decline in NCV with increase in symptoms (T-NSS) manifested by the patients.
Discussion
Chronic Kidney Disease (CKD) is a functional diagnosis characterized by an irreversible and gradually progressive decline in glomerular filtration rate (GFR). It is further complicated by an increasing inability to maintain normal levels of products of protein metabolism (urea, creatinine), normal blood pressure, hematocrit, sodium, water, calcium phosphate homeostasis, potassium and acid base balance.
Among the myriad complications manifesting in ESRD, polyneuropathy has been recognized as the most common complication. Uremic neuropathy presents as distal painless, progressive, symmetrical, sensorimotor polyneuropathy; as the neuropathy worsens the symptoms including dysesthesia manifest and ascend. There is segmental demyelination and axonal degeneration in peripheral nerves. Peripheral neuropathy complicating CKD was described as early as 1962 by Arthur K. Asbury. In his own words “The fact that chronic renal failure may be associated with polyneuropathy is not generally appreciated and is practically undocumented in the medical literature. In the briefest possible terms, the neurological disease may be defined as follows: It began with painful burning sensations of the feet, followed by a slowly progressive numbness and weakness. The feet and legs were affected more than the hands and arms, and the distal segments more than the proximal.” Our understanding of this particular disease entity has evolved considerably since he had opened a Pandora’s Box five decades ago and provided an insight into peripheral neuropathy in CKD patients.Citation12
Despite the high prevalence of peripheral neuropathy in CKD, the pathophysiology remains unknown. Extensive studies have been carried out in this direction, yet we have been unable to decipher the genesis of this disease entity. Our early understanding of peripheral neuropathy revolved around the theory of “middle molecule hypothesis” proposed in 1980s which hypothesized that substances with a molecular weight of 300–12,000 Da were the cause of uremic neuropathy.Citation13–16 A number of these middle molecules accumulate in ESRD, including β-2 microglobulin and parathyroid hormone, and these were held responsible for inducing neuropathy. However, lack of evidence that any of these substances are actually neurotoxic, quickly put this theory out of favor. Potassium satisfies all the criteria that have been proposed for a substance to be truly regarded as a uremic neurotoxin.Citation17 It has been demonstrated to causes neurological dysfunction in both humans and experimental animals. It is also a critical determinant of axonal resting membrane potential. Furthermore; substantiating the role of K+, excitability studies have demonstrated that a direct relationship exists between serum levels of K+ and neurophysiological abnormalities in patients of ESRD and that removal of K+ leads to improvement in nerve function.Citation18 Recently it has been hypothesized that, axonal depolarization may also be induced through alteration in the function of the electrogenic energy-dependent Na/K pump and inhibition of the Na/K pump by uremic neurotoxins has also been proposed as the mechanism underlying membrane depolarization in uremic patients.Citation19–21
As clinicians we face two problems while managing patients of CKD with peripheral neuropathy; establishing the existence of involvement of peripheral nervous system and ascertaining its nature. It becomes a necessity to perform a number of procedures such as biochemical tests, CSF examination, electromyography (EMG), nerve muscle biopsy, and electrophysiological studies (EPS). Measurements of nerve excitability, which provide information about membrane potential and biophysical properties of peripheral axons, have been used to study peripheral nerves in patients with neuropathy and have provided information about disease pathophysiology. Among all the aforementioned investigations, nerve conduction studies have been found to be the most sensitive detector of neuropathy, especially during its asymptomatic phase. This non-invasive procedure provides a definite evidence of sub-clinical neuropathy and often precedes either signs or symptoms of uraemia.Citation22
The present study was conducted on patients of chronic kidney disease, who had a persistently elevated serum creatinine levels greater than 2 mg/dL. Although the prevalence of neuropathy has been classically described innumerable times in medical literature; especially in patients of ESRD on maintenance hemodialysis, we excluded patients on hemodialysis in our study in an attempt to negate any beneficial effect of dialysis on the peripheral nerve dysfunction. Our objective was to assess the prevalence of peripheral neuropathy in the early as well as advanced stages of CKD and to correlate the extent of nerve damage to renal dysfunction as measured by serum creatinine levels.
The patients were subjected to detailed history and meticulous examination. Neurological history and examination were undertaken, and neuropathic symptoms were graded using the NSS. Each symptom received a score of 1, and the total number of symptoms present in each subset was summed to give a T-NSS. The maximum possible T-NSS was 17.Citation10,Citation11 Majority of the patients had at least one neurological symptom (70%), as compared against the minority (30%) who were asymptomatic. 49% of the patients had a T-NSS of ≥2 while maximum score of 5 was elicited in 9% of the patients (mean T-NSS, 1.72 ± 1.61).
Krishnan et al. in their study on 12 ESRD patients graded the neuropathic symptoms using modified NSS. Each symptom received a score of 1, and the number of symptoms present in each subset was summed to give a T-NSS. The maximum possible T-NSS was 9. All 12 patients reported symptoms of neuropathy (mean T-NSS, 1.9 ± 0.2).Citation23 Laaksonen et al. staged the clinical severity of uremic neuropathy in 21 CKD patients, using a modified version of the NSS and combined this assessment with results of nerve conduction studies. The neuropathy symptoms were evaluated using a modified version of the NSS developed by Dyck. Symptomatic neuropathy (T-NSS ≥2) was observed in 13 patients among the study group (61%).Citation2 The observation in our study is consistent with the findings of Krishnan et al. and Laaksonen et al. Furthermore, we assessed the relation of T-NSS with the severity of renal dysfunction, and it was observed that T-NSS increased steadily with increase in serum creatinine levels.
In this study it was observed that 70% of the patients had uremic polyneuropathy. Meticulous neurological examination coupled with nerve conduction studies revealed a mixed sensory motor polyneuropathy, which is characteristic of uremic polyneuropathy. 30% of the patients in the study had no neuropathy. The severity of neuropathy in the present study was staged using a modified form of a previously devised system for classification of diabetic polyneuropathy.Citation11 Among patients with neuropathy, 6% had asymptomatic neuropathy with ≥2 abnormal NCV and T-NSS of 0. Symptomatic neuropathy, albeit non-disabling, was observed in 51% of the patients, while disabling neuropathy was seen in 13% of the patients. Our findings are in agreement with numerous studies which estimate the prevalence of peripheral neuropathy to 70–100% in patients of CKD.Citation1–3 Although we did exclude patients on hemodialysis, and deferred from including patients with exclusively advanced renal failure, our results did add up to the observations in the previous studies conducted on patients of ESRD on hemodialysis. This probably indicates that peripheral nerve dysfunction does begin early in the course of CKD as contrary to the popular hypothesis which postulates this entity to be limited to advanced renal failure (ESRD).Citation4
In the study by Krishnan et al., according to the neuropathy staging system, 8% had no evidence of neuropathy, 58% had stage 2, and 33% had stage 3. Similarly in the study conducted by Laaksonen et al., 81% of the patients among 21 had peripheral neuropathy. Stage 1 neuropathy was seen in 19%, stage 2 in 48%. and stage 3 in 14% of the patients.Citation2,Citation23 Tilki et al. studied 42 patients with CKD (30 on HD and 12 on CAPD). NCS showed abnormality indicating polyneuropathy in 24 out of 25 patients with clinical neuropathy signs and in 17 out of 17 patients with no clinical signs. It was thus concluded that in subclinical conditions, NCS is useful to detect the abnormalities in peripheral nerves of the uremic patients under chronic dialysis.Citation3 The observations of these studies are consistent with the results of our study.
We did a subgroup analysis to assess the implications of raising serum creatinine, which acted as a surrogate marker for worsening renal dysfunction, on prevalence of peripheral neuropathy. The patient population was divided into three different groups (A; B; C), on the basis of serum creatinine values (2–3.4; 3.5–4.9; ≥5 respectively). It was observed that the prevalence of peripheral neuropathy increased with raise in serum creatinine. Prevalence rate increased from 35% in group A to 89% and 100% in group B and C respectively, and was extremely significant (p < 0.0001). These observations suggest that although prevalent in early renal dysfunction, a significant proportion of nerve disease develops in advanced renal dysfunction. Majority of the studies conducted on peripheral neuropathy have limited the presence of this entity to patients of advanced renal dysfunction only.Citation1–4 Although these studies do provide us an insight into the patho-physiology of peripheral neuropathy, limiting the study subjects to patients of ESRD on hemodialysis, fails to provide any information regarding the burden of this disease in patients of early renal dysfunction not requiring hemodialysis. Our study is in agreement with these previous studies, that a large proportion of peripheral neuropathy is limited to advanced CKD. But we would also like to add that a significant if not major chunk of cases of peripheral neuropathy do occur during the evolving phase of CKD.
Neurophysiological studies have been used extensively in the study of peripheral neuropathy of CKD. Although they fail to provide further insight into disease pathophysiology, NCS remain the gold standard for the clinical assessment of neuropathy.Citation22 Measurements of action potential amplitude, latency, and NCV provide information on the number of conducting fibers and the conduction velocity of the fastest. Even though these data do not always correlate well with a patient’s clinical status, it does help detecting early changes with regard to nerve damage even in asymptomatic patients. Combined with a meticulous neurological examination, it is a powerful tool in early detection and classification of peripheral neuropathy. Recently, novel nerve excitability techniques have been adapted for clinical use. They provide an indirect assessment regarding the activity of a variety of axonal ion channels, energy dependent pumps and ion exchange processes activated during the process of impulse conduction. Over recent years, nerve excitability properties have been explored in a diverse range of neurological conditions including length-dependent uremic neuropathy. These studies have examined changes in nerve function that occurred in CKD patients before, during, and after a single session of hemodialysis. Measures of nerve function were also assessed in relation to changes in serum levels of potential neurotoxins, including potassium, urea, and middle molecules such as parathyroid hormone and β2-microglobulin.Citation1,Citation24,Citation25
Conduction velocity has remained one of the best measurements of peripheral nerve function. In our study nerve conduction velocity was measured in median, ulnar, peronea, l and tibial nerves. Although conduction velocity was relatively preserved (mean NCV; median nerve, 51.34 ±6.07; ulnar nerve, 53.04 ± 5.91; peroneal nerve, 44.72 ± 6.14; tibial nerve, 44.20 ± 5.17; respectively), NCV in each of the aforementioned nerves decreased with raise in serum creatinine. There was a significant correlation between these two parameters suggesting that peripheral neuropathy worsened with rise in serum creatinine levels.
Makkar et al., studied somatosensory evoked potentials, sensory, and motor nerve conduction velocity in 25 patients of chronic kidney disease and the results were compared with 15 healthy persons. Sensory nerve conduction velocity was reduced in 11/25 patients; average reduction being 18 m/s (highly significant, p < 0.001); while motor nerve conduction velocity was reduced in 11/25 patients, average reduction being 20 m/s (highly significant, p < 0.001).Citation26 In a study by Ogura et al., nerve conduction studies of the lower extremities were done in 70 hemodialysis patients and 20 normal volunteers. Compared with that in normal volunteers, motor conduction velocity in the tibial nerve of patients was reduced significantly (p < 0.05); sensory nerve conduction velocity in the medial plantar nerve was also reduced significantly (p < 0.05).Citation5
As mentioned earlier, majority of our patients were symptomatic with T-NSS of at least 1 in 70% of the patients. Correlation was noted between clinical symptoms and NCV. Patients with a greater number of neuropathic symptoms showed more severe reduction in NCV from their normal range. These findings suggest a direct relation between excitability abnormalities and neuropathic symptoms. The observations in our study mirror the findings of studies conducted earlier in this regard. Krishnan et al. studied the excitability properties of lower limb motor nerves (common peroneal nerve) in 14 patients with end-stage renal disease treated with hemodialysis. They reported a significant correlation between clinical symptoms and excitability abnormalities.Citation1 Similar findings were reported in a study where consecutive patients with ESRD receiving hemodialysis were studied. It was observed that patients with a greater number of neuropathic symptoms showed more severe changes in this excitability parameters.Citation23
Peripheral neuropathy is one of the most commonly prevalent complications in CKD especially in its advanced stage (ESRD). Although the exact patho-physiological mechanism leading to this now common entity is not completely understood, despite extensive on-going research, it is imperative that every treating physician has in-depth knowledge regarding this disease process. Neuropathy, although not fatal, can be extremely distressing among patients of CKD and leads to a decreased sense of wellbeing. Hence, along with prevention and management of life-threatening complication of CKD, early detection of peripheral neuropathy must be a priority among physicians and nephrologists alike, who deal with patients of CKD on day to day basis. Although recent advances in neurophysiological studies and nerve excitability techniques has radically changed our perception of neuropathy as a whole, our data suggest that NCV testing when complimented with meticulous neurological assessment can provide invaluable input in daily clinical practice. These tests apart from helping us detect neuropathy in advanced renal dysfunction; can also detect the disease in largely asymptomatic patients which avoids the necessity to order for detailed neurophysiological investigation, thus being extremely cost-beneficial. In a country like India, where significant proportion of patients of CKD are denied access to comprehensive health care primarily due to monetary constraints; a simple inexpensive testing modality such as NCV can provide a wealth of information. Thus, it helps in better resource management and direction of funds toward advanced patient care and management of more severe complications of CKD.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Bostock H, Kiernan MC. Altered motor nerve excitability in end-stage kidney disease. Brain. 2005;128:2164–2174
- Laaksonen S, Metsarinne K, Voipio-Pulkki LM, Falck B. Neurophysiologic parameters and symptoms in chronic renal failure. Muscle Nerve. 2002;25:884–890
- Tilki HE, Akpolat T, Coskun M, Stalberg E. Clinical and electrophysiologic findings in dialysis patients. J Electromyogr Kinesiol. 2009;19(3):500–508
- Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004;107:1–16
- Ogura T, Makinodan A, Kubo T, Hayashida T, Hirasawa Y. Electrophysiological course of uraemic neuropathy in hemodialysis patients. Postgrad Med J. 2001;77(909):451–454
- Bolton CF, Baltzan MA, Baltzan RB. Effects of renal transplantation on uremic neuropathy. A clinical and electrophysiologic study. N Engl J Med. 1971;284:1170–1175
- Bolton CF. Electrophysiologic changes in uremic neuropathy after successful renal transplantation. Neurology. 1976;26(2):152–161
- Bolton CF. Peripheral neuropathies associated with chronic renal failure. Can J Neurol Sci. 1980;7:89–96
- Asbury AK. Neuropathies with chronic renal failure, hepatic disorders, chronic respiratory insufficiency, and critical illness. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, eds. Peripheral Neuropathy. Philadelphia: WB Saunders; 1993:1251--1265
- Dyck PJ, Sherman WR, Hallcher LM, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980;8:590–596
- Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve. 1988;11:21–32
- Asbury AK, Victor M, Adams RD. Uremic polyneuropathy. Arch Neurol Psychiatr. 1963;8:413–428
- Babb AL, Popovich RP, Christopher TG, Scribner BH. The genesis of the square meter-hour hypothesis. Trans Am Soc Artif Intern Organs. 1971;17:81–91
- Vanholder R, De Smet R, Hsu C, Vogeleere P, Ringoir S. Uremic toxicity: the middle molecule hypothesis revisited. Semin Nephrol. 1994;14:205–218
- Furst P, Zimmerman L, Bergstrom J. Determination of endogenous middle molecules in normal and uremic body fluids. Clin Nephrol. 1976;3:178–188
- Man NK, Granger A, Rondon-Nucete M, et al. One year follow-up of short dialysis with a membrane highly permeable to middle molecules. Proc Eur Dial Transplant Assoc. 1973;10:236–246
- Bostock H, Walters RJ, Andersen KV, Murray NM, Taube D, Kiernan MC. Has potassium been prematurely discarded as a contributing factor to the development of uraemic neuropathy? Nephrol Dial Transplant. 2004;19:1054–1057
- Rakowski RF, Gadsby DC, De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage clamped, internally dialyzed squid giant axon. J Gen Physiol. 1989;93:903–941
- Nielsen VK. The peripheral nerve function in chronic renal failure. V. Sensory and motor conduction velocity. Acta Med Scand. 1973;194:445–454
- Bostock H, Burke D, Hales JP. Differences in behavior of sensory and motor axons following release of ischemia. Brain. 1994;117:225–234
- Kiernan MC, Bostock H. Effects of membrane polarization and ischemia on the excitability properties of human motor axons. Brain. 2000;123:2542–2551
- Krishnan AV, Pussell BA, Kiernan MC. Neuromuscular disease in the dialysis patient: An update for the nephrologist. Semin Dial. 2009;22(3):267–278
- Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Kiernan MC. Sensory nerve excitability and neuropathy in end stage kidney disease. J Neurol Neuro Surg Psychiatry. 2006;77(4):548–551
- Krishnan AV, Colebatch JG, Kiernan MC. Hypokalemia induces activity-dependent conduction block. Neurology. 2005; 65:1309–1312
- Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Bostock H, Kiernan MC. Neuropathy, axonal Na/K pump function and activity-dependent excitability changes in endstage kidney disease. Clin Neurophysiol. 2006;117:992–997
- Makkar RK, Kochar DK. Somatosensory evoked potentials (SSEPs); sensory nerve conduction velocity (SNCV) and motor nerve conduction velocity (MNCV) in chronic renal failure. Electromyogr Clin Neurophysiol. 1994;34(5):295–300