Abstract
Objective: To investigate changes of adiponectin and its receptors (Adipo R) in rats following chronic renal failure. Methods: Male SD rats were randomly divided into two groups: control group and chronic renal failure (CRF) group. The CRF group were gavaged with adenine (300 mg/kg/d) for 4 weeks and the control group with drinking water. All rats were anesthetized at 2nd or 4th week and blood and urine samples were collected for detection of renal function, 24 h urine protein and adiponectin concentration. Renal tissues were also collected for HE staining, immunohistochemistry and RT-PCR screening. Results: Compared with control group, the serum concentrations of urea and creatinine, 24 h urine protein excretion in the CRF group were significantly higher at 2nd week and further increased at 4th week (p < 0.05). The adiponectin levels in serum and urine in the CRF group were significantly higher than those of control group (p < 0.01). The renal expressions of Adipo R1 and Adipo R2 in CRF group were also significantly increased compared to control group (p < 0.01). The increased expressions of Adipo R1 and Adipo R2 were positively related to the adiponectin levels in serum, urine, and 24 h urine protein. Conclusion: The significant changes in expression of adiponectin and its receptors in rat CRF model could be an adaptive response that may provide the basis to understand pathological changes in chronic kidney disease.
Introduction
Being specifically expressed by adipocytes, adiponectin is a polypeptide hormone, which shares structural homology with type VIII and X collagen and complement factor C1q. Adiponectin is the unique hormone and involved in various physiological functions or pathological process. Adiponectin performs its biological effect through the adiponectin receptors (Adipo R), including Adipo R1 and Adipo R2. Adipo R1 is widely expressed in muscle tissue, while Adipo R2 has strongest expression in the liver. Adipo R displays its biological effects through the adenosine monophosphate-activated protein kinase (AMPK) pathway.Citation1 It has been confirmed that adiponectin has many beneficial effects on the general population by regulating glucose and fat metabolism, energy balance, attenuating insulin resistance, preventing atherosclerosis, decreasing inflammatory reactions, restraining interstitial fibrosis and protecting cardiovascular system.Citation2 Scherer and colleagues reported that an acute increase of circulating adiponectin triggers a transient decrease in basal glucose level by inhibiting both the expression of hepatic gluconeogenic enzymes and the rate of endogenous glucose production in both wild-type and type 2 diabetic mice, and they proposed that adiponectin sensitizes insulin effects.Citation3
A number of clinical studies have observed that adiponectin levels in serum of CRF patients significantly increased with the progression of chronic kidney disease (CKD).Citation4 The reduction of degradability and scavenging ability was one of the main factors for the increase of adiponectin levels. Besides, adiponectin is associated with many risk factors for metabolic disorders, such as insulin sensitivity, triglycerides, high-density lipoprotein cholesterol, obesity, microalbuminuria and inflammation.Citation5 Adiponectin levels are also associated with risk factors for cardiovascular disease.Citation6 Increased evidences indicate that adiponectin has beneficial effects in the cardiovascular system through modulation of signaling molecules and might be a promising therapeutic target for cardiovascular diseases.Citation7
In adiponectin knockout mice, exogenous adiponectin can attenuate proteinuria and oxidative stress level, reduce podocyte injury and activate AMPK. A hypothesis concerning adiponectin receptor resistance to overstimulation by increased plasma adiponectin concentration in CKD remains unconfirmed.Citation8 The functions and mechanism of adiponectin and its receptors under CRF status have not well been studied to date and further study is necessary. Increasing plasma adiponectin levels or the activity of the receptors (including thiazolidinedione, renin--angiotensin system inhibitor or calcium channels blocker) for the specific population may become a new therapeutic target and may play a significant role in clinical intervention and treatment.Citation9,Citation10
In this study, we have studied changes in adiponectin levels and Adipo R expression of renal tissues in rat CRF model. We have also discussed the observed correlation between Adipo R and adiponectin levels to provide the basis for understanding the function and mechanism of adiponectin following CRF.
Materials and methods
Materials and main reagents
Eight-week clean Male SD rats, weighing about 170–200 g, were provided by the Laboratory Animal Center at Medical Center of Nantong University (Nantong, China). Following reagents were used for the experiment: Adenine (Sigma Chemical Company, St Louis, MO), Trizol (Invitrogen, Carlsbad, CA), Adiponectin monoclonal antibodies and Adipo R1, Adipo R2 polyclonal antibodies (Abcam, Cambridge, MA), horseradish peroxidase (HRP)-conjugated anti-goat IgG (GBI, Cincinnati, OH). Rat Adiponectin ELISA Kit (Shanghai Xitang Biological Technology Co., Ltd, Shanghai, China) and PCR Primers (Shanghai Sangon Company, Shanghai, China) were also applied in the study.
Preparation of CRF rat models
Male SD rats were randomly allocated into two groups: control group (10 rats) and CRF group (20 rats). The rats maintained under a controlled environment, at 20 ± 2 °C, 12/12 h light/dark cycle, with food and water ad libitum. The use of animals was conformed to the regulations and standards of Jiangsu Animal Management Committee and the animal experiments were certified as well. After one week adaptive feeding, as referred to the methods reported by Yokozawa,Citation11 rats in the CRF group were gavaged with adenine (300 mg/kg/d) for 4 weeks and those in the control group with drinking water. The rats were then weighed and adenine dosage adjusted every four days. Half of the rats from each group (10 from the CRF group and 5 from the control group) were anesthetized and then blood and urine samples collected for detection of renal function, 24 h urinary protein and adiponectin concentration at 2nd and 4th week, respectively.
Renal function, urine protein and adiponectin concentration testing
The rats were placed into metabolic cages, and 24-h urine samples were collected from all the subjects and stored at −80 °C. Then the rats were anaesthetized by intraperitoneal injection of 10% chloralhydrate (3.0 mL/kg) and blood samples were taken from their hearts. Sera were isolated by centrifuge and kept at −80 °C before testing. Urinary proteins were determined using Bradford's method, serum creatinine concentrations were assayed by standard enzymatic methods, and blood urea nitrogen (BUN) concentration was assessed by urease-GLDH method. Plasma and urinary adiponectin levels were measured by enzyme-linked immunosorbent assay (ELISA).
Renal histopathological examination
Fresh kidney tissues were fixed in formaldehyde overnight, and then dehydrated in graded ethanol, cleared with xylene, paraffin-embedded, sectioned and finally stained with hematoxylin-eosin. The glomeruli in five non-overlapping fields of the cortex were examined at low power magnification (200×) while histopathological changes were observed at high power magnification (400×).
Immunohistochemistry testing of Adipo R in Kidney tissues
We dewaxed and rehydrated the unstained sections through a series of descending grades of alcohol to distilled water. The sections were washed with 0.01 M citrate buffer (pH 6.0), blocked with 3% hydrogen peroxide, 5% bovine serum albumin (BSA), and then incubated overnight at 4 °C with primary antibodies. After incubation with HRP-goat anti-rabbit IgG and peroxidase complex, immunoreactive sites were finally visualized by diaminobenzidine (DAB) reaction and hematoxylin counter stain. Five non-overlapping high-power fields (HPF, × 400 magnification, visual field area) were randomly selected for every section. Brown yellow stained substances were considered as positive expressions of Adipo R1 and Adipo R2. HPIAS-2000 image analysis software (Wuhan, China) was adopted as semi-quantitative image analysis method for immunohistochemical slide images. Average absorbance was calculated as the relative content of the positive expressions.
Real-time quantitative PCR detection of Adipo R1 and Adipo R2 mRNA expression
Total RNA was extracted from kidney tissues using the Trizol method. cDNA was synthesized by reverse transcription (RT). Primer sequences used for real time PCR were as follows: Adipo R1: the forward primer 5′-CTGCTGGTCCTTCACAGACA-3′ and the reverse primer 5′-CATCCGCCAGGTTACAAAGT-3′ (172 bp amplification product). Adipo R2: the forward primer 5′-ACCCACAACCTTGCTTCATC-3′ and the reverse primer 5′-GCTAGCCATGAGCATTAGCC-3′ (233 bp amplification product). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): the forward primer 5′-AGACAGCCGCATCTTCTTGT-3′ and the reverse primer 5′-CTTGCCGTGGGTAGAGTCAT-3′ (207 bp amplification product). Real-time quantitative PCR was carried out using 25 μL reaction volumes, under the following thermo cycling conditions: 95 °C 90 s, 95 °C 5 s, 58 °C 30 s, for 40 cycles and 3 repetitions. ABI 7500 Software V 2.0.4 (Grand Island, NY) was used to analyze the amount of targets relative to GAPDH housekeeping gene.
Statistical analysis
All the dosage information was represented by . t Test was adopted to examine comparison between different time points in the two groups observed. Multiple groups were compared using single factor analysis of variance (ANOVA) and the relationship between double factors was performed using Spearman rank correlation analysis. All the statistical analyses were performed using SPSS 17.0 (Chicago, IL).
Results
Changes in general conditions and kidney functions for the two groups
After gavaging the rats with adenine for 2–4 weeks, rats in CRF group gradually displayed anorexia, increased urination, fatigue, lethargy, chills, pallor on auricles and puffiness in the eyelids. Two rats died after building the models, one in the 3rd week and the other in the 4th week. At the 2nd week of observation, the kidney function test for the CRF group showed significant increases (both BUN and Scr: p < 0.01) compared to the control group, where urine protein excretions raised (p < 0.01) and were more obvious at the 4th week (p < 0.01) ().
Table 1. Changes of renal function and urinary protein at different time points ().
Increase of serum and urine adiponectin levels in CRF rat
At the 2nd week, serum and urine adiponectin levels in CRF group were significantly higher than those of the control group (p < 0.01), and increased further at the 4th week (p < 0.01) ().
Table 2. Changes of adiponectin levels in serum and urine at different time points ().
Pathological changes
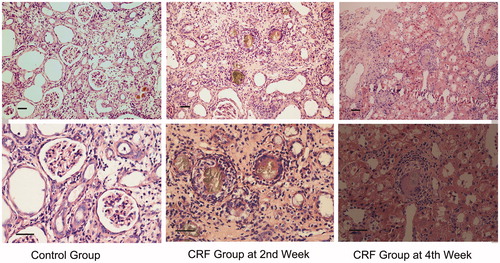
Kidney tissues from the control group appeared reddish brown and lustrous. The renal capsule was easy to peel off and the cut surfaces revealed sharp corticomedullary demarcation. The number of glomeruli was about 18 ± 5 per field in the cortex at high magnification and low power magnification as stained with HE. Structures of the glomeruli and renal tubules were normal, without obvious proliferation of fibrous tissues. In the CRF group, kidneys displayed grey white and granular surface. The renal capsule adhered to renal parenchyma and was difficult to peel off. Stained sections showed unclear corticomedullary demarcation. The sizes of kidneys decreased at the 4th week compared to the 2nd week. The number of glomeruli at the 2nd week was about 14 ± 3 per field in the cortex, at low magnification, as stained with HE. Glomerular mesangium including mesangial cells and matrix showed proliferation and expanding. Yellow-brown accumulation of adenosine appeared in the glomeruli and renal tubules. Tubular necrosis and interstitial inflammatory cell infiltration were also observed and characteristic pathological changes as acute kidney injury seen. Besides, fibrous tissues proliferated irregularly in the mesenchyme ().
Expressions of Adipo R1 and Adipo R2 in the renal tissue
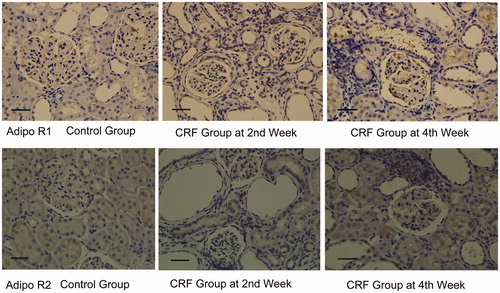
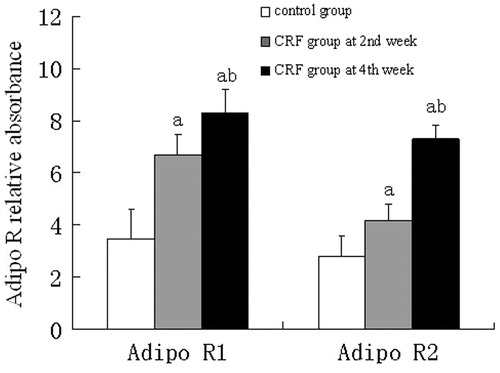
Immunohistochemistry showed that both Adipo R1 and Adipo R2 were expressed in the glomerular endothelial cells and renal tubular epithelial cells from the control group, however, the expression levels were significantly higher in the CRF group, when compared to the control group (p < 0.01) ( and ).
Adipo R1 and Adipo R2 mRNA expressions
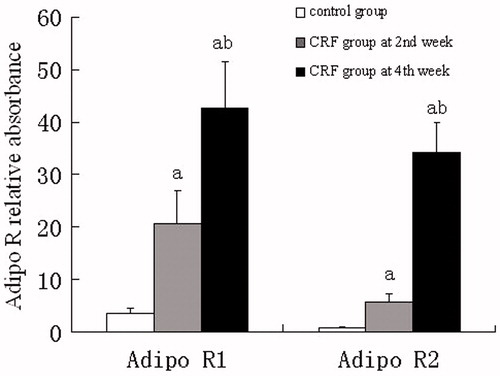
Real-time fluorescence quantitative PCR analysis showed that mRNA expression of Adipo R1 and Adipo R2 in kidney tissues from the CRF group was more abundant than those from the control group at the 2nd or 4th week (p < 0.01). The expression level of Adipo R1 mRNA was highest at the 2nd week while that of Adipo R2 at the 4th week ().
Correlation of mRNA expression levels of Adipo R1 and Adipo R2 in the renal tissue with amount of serum/urine adiponectin and urine protein of the CRF group
Adipo R1 mRNA showed positive correlation with serum and urine adiponectin and also 24 h urine protein (r = 0.72, p = 0.0048; r = 0.65, p = 0.0071; r = 0.52, p = 0.0079; respectively). There were the same tendency with Adipo R2 mRNA (r = 0.68, p = 0.0074; r = 0.59, p = 0.0071; r = 0.49, p = 0.0173; respectively).
Discussion
Adiponectin belongs to adipocytokine family. It was firstly separated from rat adipocyte by Schererin. Human adiponectin gene is located in the chromosome 3q27 and consists of 244 amino acids. Its relative molecular weight is 30 kD, accounting for about 0.01% of the human plasma protein.Citation12
CRF is the common outcome of primary or secondary renal diseases, of which cardio-cerebral vascular diseases are the most important complications and leading causes of death. Moreover, CRF is closely related to lipid metabolic disorders.Citation13
Findings from our study show that both Adipo R1 and Adipo R2 were expressed in the kidneys of normal SD rats. As for the CRF group, their serum and urinary adiponectin concentrations increased with time. Adipo R1 and Adipo R2 expressions in kidney tissues from the CRF group increased with time as well. When the renal damage became more and more severe, expression levels of Adipo R1 and Adipo R2 mRNA and adiponectin protein also increased as a result. In addition, the expression level of Adipo R1 at the 2nd week was significantly higher than that of Adipo R2 which had significant increase at the 4th week. Correlation analysis showed that when the level of adiponectin protein increased with time, its receptor’s expression also increased.
Moreover, a correlation between the increase of Adipo R expression and urinary protein excretion was established. Through type 2 diabetic rat models, Cammisotto and some experts found that renal distal tubules and thick ascending limb of loop of Henle (TAL) epithelial cells had expressions of Adipo R1, AMPK α1, AMPK α2 and β2 subunits. They found out that when adiponectin expression level became higher, its receptor’s expression level increased at the same time.Citation14 The findings in those articles have some resemblances with our findings. Adiponectin induced the AMPK activity of renal tubular epithelial cells in a time-dependent manner and decreased monocyte chemoattractant protein-1 to display protective function.Citation15 Increased proteinuria may cause endothelial and epithelial cell injury, and may also have a positive effect on adiponectin, by a feedback mechanism, as well as Adipo R expression.Citation16
Shen's research showed that,Citation17 urinary adiponectin in patients with proteinuric renal disease was mainly in the form of globular or trimeric adiponectin, and acts directly on kidney tissues through Adipo R1. The above literature may explain some findings in our study, especially the correlation between the increase in urinary protein in the CRF group and increase in Adipo R expression with time. However, among the published literatures we retrieved, changes of Adipo R1 and Adipo R2 mRNA and protein expression levels in CRF rats’ models were rarely involved. Yaturu found that in CRF patients,Citation18 glomerular filtration rate was negatively associated with urinary adiponectin and increase of plasma adiponectin, which was not caused by reduction of excretion by decreasing glomerular filtration rate. Since adiponectin could adjust the balance between adhesion molecules and other substances that form the endothelium, the increase of adiponectin levels and renal Adipo R expressions might be a result of a kind of adaptive and self-protective response to metabolic disorders.Citation19 Higher adiponectin level may be needed to resist the state of CRF, while cardiovascular protection effects of adiponectin were partly inhibited during CRF progression and its mechanism were as yet unknown.Citation20
Adiponectin has been suggested as a potential biomarker in CKD progression.Citation21 However, the studies linking adiponectin and renal function have two potential limitations. First, the results and conclusions of these studies have been inconsistent and contradictory.Citation22–25 Second, most of the studies investigating the relationship between adiponectin and kidney function have been conducted in persons with existing metabolic disorders (e.g., diabetes).
In summary, our study has found out a timely dependent increase of Adipo R1 and Adipo R2 mRNA and adiponectin protein expression levels in renal tissue of rat CRF model, which is also associated with timely increase in serum and urinary adiponectin and urine protein excretion. Further study on the functions of adiponectin during CRF in humans is of great importance in order to find targets for prevention and treatment CKD and its complications.
Declaration of interest
This work was supported by Science and Technology Program of Jiangsu Provincial Health Department, Grant number: H201016. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
We thank Mr. Wu Ya-jun of Research Institute of Nephrology for technical assistance.
References
- Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295
- Santaniemi M, Kesaniem YA, Ukkola O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur J Endocrinol. 2006;155:745–750
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953
- Kamimura MA, Canziani ME, Sanches FR, et al. Variations in adiponectin levels in patients with chronic kidney disease: a prospective study of 12 months. J Bras Nefrol. 2012;34(3):259–265
- Guebre-Egziabher F, Bernhard J, Funahashi T, Hadj-Aissa A, Fouque D. Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol Dial Transplant. 2005;20:129–134
- Cui J, Panse S, Falkner B. The role of adiponectin in metabolic and vascular disease: a review. Clin Nephrol. 2011;75:26–33
- Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J. 2009;73:608–614
- Adamczak M, Chudek J, Wiecek A. Adiponectin in patients with chronic kidney disease. Semin Dial. 2009;22:391–395
- Zoccali C, Mallamaci F. Obesity, diabetes, adiponectin and the kidney: a podocyte affair. Nephrol Dial Transplant. 2008;23:3767–3770
- Nakagawa N, Yao N, Hirayama T, et al. Potential impact of renin--angiotensin system inhibitors and calcium channel blockers on plasma high-molecular-weight adiponectin levels in hemodialysis patients. Hypertens Res. 2011;34:592–598
- Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron. 1986;44:230–234
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749
- Fan YP, Yu Y. Intervention of dyslipidemia in chronic kidney disease. Chinese J Nephrol. 2010;26:233–237
- Cammisotto PG, Bendayan M. Adiponectin stimulates phosphorylation of AMP-activated protein kinase alpha in renal glomeruli. J Mol Histol. 2008;39:579–584
- Zhou Z, Wu XY, Yao T, Gao P, Yu WL, Yang GM. Protective effect of adiponectin and its receptors in diabetic nephropathy rats. Chinese J Nephrol. 2010;26:702–707
- Fujita H, Morii T, Koshimura J, et al. Possible relationship between adiponectin and renal tubular injury in diabetic nephropathy. Endocr J. 2006;53:745–752
- Shen YY, Hughes JT, Charlesworth JA, Kelly JJ, Peake PW. Adiponectin is present in the urine in its native conformation and specifically reduces the secretion of MCP-1 by proximal tubular cells. Nephrology (Carlton). 2008;13:405–410
- Yaturu S, Reddy RD, Rains J, Jain SK. Plasma and urine levels of resistin and adiponectin in chronic kidney disease. Cytokine. 2007;37:1–5
- Malyszko J, Malyszko JS, Brzosko S, Wolczynski S, Mysliwiec M. Adiponectin is related to CD146, a novel marker of endothelial cell activation/injury in chronic renal failure and peritoneally dialyzed patients. J Clin Endocrinol Metab. 2004;89:4620–4627
- Stenvinkel P. Adiponectin in chronic kidney disease: a complex and context sensitive clinical situation. J Ren Nutr. 2011;21:82–86
- Devarajan P. The use of targeted biomarkers for chronic kidney disease. Adv Chr Kid Dis. 2010;17:469–479
- Risch L, Saely C, Hoefle G, et al. Relationship between glomerular filtration rate and the adipokines adiponectin, resistin and leptin in coronary patients with predominantly normal or mildly impaired renal function. Clin Chim Act. 2007;376:108–113
- Kawamoto R, Tabara Y, Kohara K, et al. Serum high molecular weight adiponectin is associated with mild renal dysfunction in Japanese adults. J Athero Thromb. 2010;17:1141–1148
- Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16:1091–1098
- Lin J, Hu FB, Curhan G. Serum adiponectin and renal dysfunction in men with type 2 diabetes. Diabet Care. 2007;30:239–244