Abstract
Background: Cisplatin is a potent anticancer drug, but its nephrotoxicity limits the clinical use of it. To reduce the Cisplatin-induced nephrotoxicity, various interventions have been implicated. The aim of this study was to examine whether preconditioning with normobaric hyperoxia would prevent Cisplatin-induced nephrotoxicity in patient with solid tumor. Methods: In a prospective study, 80 adult patients with solid tumor who were treated with Cisplatin between February 2011 and December 2011 were included. Forty-three patients were exposed to pure oxygen via non-rebreathing reservoir mask which increased the provided oxygen rate to 60% oxygen for 2 hours at 48, 24, and 6 hours before intravenous administration of Cisplatin and 37 patients received only Cisplatin as a control group. Estimated glomerular filtration rate (eGFR) calculated in all patients on day 1 before and on days 1, 3, 6, 30 after Cisplatin exposures. Results: Patients treated with Cisplatin and 60% oxygen showed a mild improvement in eGFR and mild reduction of serum creatinine after 30 days with statistically mild significant differences (p = 0.048). Conclusion: This study showed that normobaric and intermittent precondition of 60% oxygen prior to Cisplatin treatment had an acute transient adverse effect on renal function; however, the improvement of renal function will be seen after 30 days. Thus, it may help to prevent Cisplatin nephrotoxicity.
Introduction
Cisplatin is a broad-spectrum anticancer agent that is widely used for treatment of several human solid tumors. However, its potential renal toxicity restricts the clinical use of it.Citation1
Nephrotoxicity is the major adverse effect with irreversible renal damage in about one-third of Cisplatin-treated patients.Citation2 Despite numerous studies, the related mechanisms are not completely understood.Citation3,Citation4 The kidney accumulates Cisplatin to a greater amount compared with other organs and is the major route for its excretion. The Cisplatin accumulates in proximal tubule cells and its concentration is approximately five times more than the serum concentration, which contributes to Cisplatin-induced renal insufficiency.Citation4
Reactive oxygen species (ROS) formation and depletion of antioxidant enzymes are also involved in Cisplatin nephropathy.Citation5 Oxidative stress injury plays an important role in the pathogenesis of Cisplatin-induced nephrotoxicity and mitochondrial dysfunction.Citation3,Citation4,Citation6 Oxidative stress occurs when ROS generation overrides metabolic capacity of the antioxidant defense system, often resulting in tissue damage. Moreover, ROS directly act on tubular cell components and destroy their structure.Citation4 Increased Cisplatin-mediated production of free radicals by kidneys has been shown in whole experimental models and also in cultured renal tubular cells after Cisplatin administration.Citation3 ROS and the rise in lipid peroxidation may contribute to the pathogenesis of acute Cisplatin-induced nephrotoxicity.Citation7,Citation8 However, it is still controversial whether oxygen therapy plays a toxic role in renal injury. Exposure to high concentrations of oxygen is known to induce injury to cells, possibly due to an increased oxygen radical formation.Citation9 On the other hand, some evidences suggest that exposure to oxygen prior to Cisplatin therapy may reduce the Cisplatin-induced nephrotoxicity in animal model.Citation10,Citation11 Hypoxia and mitochondrial injury are also implicated in Cisplatin nephrotoxicity.Citation7
According to our literature review, there is no clinical human study in terms of impact of oxygen therapy before Cisplatin administration on kidney injury. This study examines whether preconditioning with normobaric hyperoxia would prevent Cisplatin-induced nephrotoxicity in patients with solid tumors.
Materials and methods
Objective
The study was undertaken to determine the role of oxygen therapy (OT) prior to chemotherapy as a protective agent against Cisplatin nephrotoxicity.
Patients
Eighty adult patients with solid tumor who were supposed to be treated with Cisplatin as a chemotherapy agent were referred to Oncology unit of Baqiyatallah University of Medical Sciences, Tehran, Iran, between February 2011 and December 2011. The study was approved by local University ethics committee, and informed consent was obtained from all participants. The patients were randomly allocated into two groups: Group I (case group) undergoing oxygen therapy (n = 43) and Group II (control group) who did not receive OT (n = 37).
summarizes demographic data of the two groups.
Table 1. Demographic characteristics of the patients receiving cisplatin with and without exposure to hyperoxia.
Inclusion and exclusion criteria
Adult patients who were candidates for chemotherapy with Cisplatin due to various malignancies were included. The patients who had chronic obstructive lung disease, pneumothorax, upper respiratory infection, fever, viral infection, history of seizure, optic nerve inflammation, history of ear surgery, and history of heavy cigarette smoking were excluded from this study. None of female patients were pregnant. None of the investigated individuals received other nephrotoxic agents.
Treatment protocol
Forty-three patients were exposed to pure oxygen via non-rebreathing reservoir mask which increased the provided oxygen rate to 60% oxygen for 2 hours at 48, 24, and 6 hours before intravenous administration of Cisplatin. Venous blood samples were obtained from all patients for serum urea nitrogen and creatinine (Cr) measurements on the day before and the 1st, 3rd, 6th, and 30th day after Cisplatin exposures. The renal function was also measured with the eGFR calculated by the Cockcroft–Gault equation [GFR = (140-age) × (Wt in kg) × (0.85 if female)/(72 × Cr)].
The chemotherapy protocol was 75 mg/m2 Docetaxel (trade name Taxotere) (first day), followed by 50 mg/m2 intravenous Cisplatin for second day. All patients received 350 mg/m2 5-Fluorouracil simultaneously with injection of Taxotere for five days. Two to three liters of normal saline were infused during Cisplatin administration days.
Statistical analysis
Statistical analysis was performed using the SPSS 17.0 program (SPSS Inc., Chicago, IL). Pre- and post-treatment values in the same groups were compared with paired samples Wilcoxon test. Pre- and post-treatment comparisons in different groups were performed using the Mann–Whitney U test and repeated measures ANOVA test.
The Fisher’s exact test was used to compare groups by categorical variables. A value of p < 0.05 was considered statistically significant.
Results
Eighty patients, 30 males and 50 females, were enrolled in the study, most of them received chemotherapy because of gastrointestinal cancers. The 43 patients allocated to oxygen preconditioning and the 37 others who served as controls were comparable regarding the base line characteristics except the blood pressure which happened to be higher in control group ().
No complications related to the oxygen therapy were detected. Two patients in control group and seven patients in case group experienced 25–30% reduction of eGFR in different points of time after injection of Cisplatin. While 8 patients in control group and 12 patients in case group showed an improvement in eGFR after 30 days (p = 0.517) ().
Table 2. Incidence of cisplatin-induced nephrotoxicity in two groups.
Renal function comparisons in pre- and post-treatment between both groups are summarized in .
Table 3. Renal function comparisons in pre- and post-treatment between both groups.
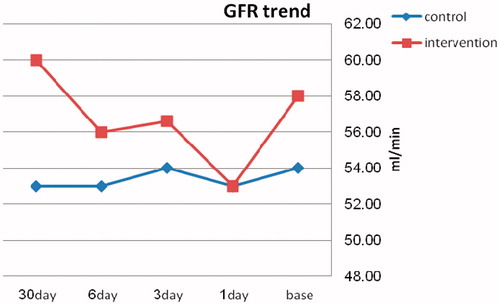
Both groups had statistically significant within subjects changes in BUN level, from the day before, through 1st, 3rd, 6th, and 30th day after Cisplatin exposure (p < 0.0001). While changes for GFR and serum Cr through the same time were not statistically significant (p = 0.43 and p = 0.35, respectively) within either groups. The comparison made between the trends of eGFR (), serum Cr, and BUN changes in Group I with Group II revealed no statistically significant difference ().
Table 4. GFR changes trend; within and between subjects variations.
Discussion
In this prospective cohort study, we investigated the use of intermittent normobaric hyperoxia preconditioning to prevent nephropathy in patients with solid tumor treated with Cisplatin. Despite the high prevalence of Cisplatin nephrotoxicity reported in many studies,Citation4 we observed just a few cases of eGFR transient reduction in study sample.
The trend toward improved GFR after 30 days (53 mL/min to 60 mL/min) is a clinically considerable finding though it might be classified as mild change from statistical view.
To our knowledge, this is the first study that indicates this effect of oxygen preconditioning against Cisplatin-induced nephrotoxicity in human. However, Rasoulian et al. recently showed that preconditioning with intermittent normobaric hyperoxia improved rat renal function after Cisplatin administration.Citation10 They also reported that preconditioning by oxygen therapy notably decreased Cisplatin-induced renal catalase activity and glutathione level. They recommended that oxygen preconditioning may lead to a delayed protective impact on Cisplatin-induced nephrotoxicity, and that enhanced renal catalase activity could be playing a role in this protective effect of hyperoxia.Citation10
In addition, hyperbaric oxygen (HBO) therapy is another approach for preventing Cisplatin nephrotoxicity. Atasoyu et al. observed that HBO therapy prior to Cisplatin administration could lead to prevention of experimental Cisplatin nephrotoxicity.Citation11
Pure oxygen at atmospheric pressure is safe if given for less than 6 hours; 70% oxygen is probably safe for 24 hours;Citation12 which makes it predictable that the 60% oxygen exposure for 2 hours per day used in this study was associated with no complications related to the oxygen therapy. Importantly, it was revealed that the protective effects of pure oxygen exposure on rat heart tissue can be seen with 80% oxygen as wellCitation13 and this may be true for other tissues such as kidney.Citation14
Some studies suggest that intermittent and prolonged normobaric hyperoxia induces brain ischemic tolerance.Citation15,Citation16 Bigdeli et al. showed the intermittent and prolonged normobaric hyperoxia resulted in increase antioxidant enzymes activities.Citation15 It is known that normobaric hyperoxia can lead to enhanced ROS generation. ROS may stimulate the activation of antioxidant enzymes including superoxide dismutase, glutathione peroxidase, catalase, and glutathione reductase, which play an important role in the brain ischemic tolerance.Citation15
Although ROS production has several theoretical adverse effects, HBO has been successfully used in experimental models of ischemic reperfusion injury including myocardium, skeletal muscle, small intestine, and the liver.Citation17 Lipid peroxidation occurs in the presence of ischemic reperfusion injury due to the release of ROS. Despite HBO can lead to ROS generation, reduction of lipid peroxidation occurs when exposed to HBO.Citation18 Rubinstein et al. demonstrated that acute ischemic renal injury is associated with increased renal oxidative stress and that HBO therapy preserves the renal function by improving the antioxidant/oxidant balance in the ischemic kidney.Citation19 Han et al. showed that HBO preconditioning decreased infarct size and improved heart function in rat myocardial infarction.Citation20 In addition, Yogaratnam et al. demonstrated that HBO preconditioning before coronary artery bypass graft surgery decreased myocardial damage, intraoperative blood loss, ICU length of stay, and postoperative complications.Citation21
Interestingly, in an experimental study, HBO therapy results in an otoprotection effect against Cisplatin-induced ototoxicity.Citation22 In a series of 89 liver transplant patients, donor hyperoxygenation before organ harvest, defined as a PaO2 value greater than 150 mmHg, was associated with good graft outcome.Citation23 It could be assumed that the hyperoxia preconditioning of donor has reduced liver ischemic reperfusion injury.
Limitations
A limitation of this study is that the numbers of patients and controls are relatively small.
Thus, further studies in a larger sample are needed to clarify whether this treatment is effective for prevention of Cisplatin nephrotoxicity.
Conclusion
This study indicated that normobaric and intermittent precondition with 60% oxygen prior to Cisplatin treatment is associated with an acute transient adverse effect on renal function, but an ultimate improvement by 30 days. Thus, the intervention could be considered as an option while trying to prevent Cisplatin nephrotoxicity.
Declaration of interest
The authors declare that there is no conflict of interest.
Acknowledgments
We express our appreciation to Baqiyatallah hospital chemotherapy ward staff.
References
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007
- Razzaque MS. Cisplatin nephropathy: is cytotoxicity avoidable? Nephrol Dial Transplant. 2007;22(8):2112–2126
- Jia Z, Wang N, Aoyagi T, Wang H, Liu H, Yang T. Amelioration of Cisplatin nephrotoxicity by genetic or pharmacologic blockade of prostaglandin synthesis. Kidney Int. 2011;79(1):77–88
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334(2):115–124
- Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in Cisplatin induced nephrotoxicity. Exp Toxicol Pathol. 2009;61(3):223–242
- Jung M, Hotter G, Vinas JL, Sola A. Cisplatin upregulates mitochondrial nitric oxide synthase and peroxynitrite formation to promote renal injury. Toxicol Appl Pharmacol. 2009;234(2):236–246
- Kawai Y, Nakao T, Kunimura N, Kohda Y, Gemba M. Relationship of intracellular calcium and oxygen radicals to Cisplatin-related renal cell injury. J Pharmacol Sci. 2006;100(1):65–72
- Satoh M, Kashihara N, Fujimoto S, et al. A novel free radical scavenger, edarabone, protects against Cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther. 2003;305(3):1183–1190
- Dennog C, Hartmann A, Frey G, Speit G. Detection of DNA damage after hyperbaricoxygen (HBO) therapy. Mutagenesis. 1996;11(6):605–609
- Rasoulian B, Jafari M, Mahbod M, et al. Pretreatment with oxygen protects rat kidney from Cisplatin nephrotoxicity. Ren Fail. 2010;32(2):234–242
- Atasoyu EM, Yildiz S, Bilgi O, et al. Investigation of the role of hyperbaric oxygen therapy in Cisplatin-induced nephrotoxicity in rats. Arch Toxicol. 2005;79(5):289–293
- Tinits P. Oxygen therapy and oxygen toxicity. Ann Emerg Med. 1983;12(5):321–328
- Tahepold P, Ruusalepp A, Li G, Vaage J, Starkopf J, Valen G. Cardioprotection by breathing hyperoxic gas-relation to oxygen concentration and exposure time in rats and mice. Eur J Cardiothorac Surg. 2002;21(6):987–994
- Rasoulian B, Mohammad hosseniakbari H, Kadkhodaee M, et al. Preconditioning with oxygen attenuates rat renal ischemia-reperfusion injury. J Surg Res. 2008;146(2):282–288
- Bigdeli MR, Rasoulian B, Meratan AA. In vivo normobaric hyperoxia preconditioning induces different degrees of antioxidant enzymes activities in rat brain tissue. Eur J Pharmacol. 2009;611(1–3):22–29
- Bigdeli MR, Khoshbaten A. In vivo preconditioning with normobaric hyperoxia induces ischemic tolerance partly by triggering tumor necrosis factor-alpha converting enzyme/tumor necrosis factor-alpha/nuclear factor-kappaB. Neuroscience. 2008;153(3):671–678
- Piper HM, Garcia-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38(2):291–300
- Muralidharan V, Christophi C. Hyperbaric oxygen therapy and liver transplantation. HPB (Oxford). 2007;9(3):174–182
- Rubinstein I, Abassi Z, Milman F, et al. Hyperbaric oxygen treatment improves GFR in rats with ischemia/reperfusion renal injury: a possible role for the antioxidant/oxidant balance in the ischemic kidney. Nephrol Dial Transplant. 2009;24(2):428–436
- Han C, Lin L, Zhang W, et al. Hyperbaric oxygen preconditioning alleviates myocardial ischemic injury in rats. Exp Biol Med (Maywood). 2008;233(11):1448–1453
- Yogaratnam JZ, Laden G, Guvendik L, Cowen M, Cale A, Griffin S. Hyperbaric oxygen preconditioning improves myocardial function, reduces length of intensive care stay, and limits complications post coronary artery bypass graft surgery. Cardiovasc Revasc Med. 2010;11(1):8–19
- Yassuda CC, Righetti AE, Cury MC, Hyppolito MA, Oliveira JA, Feres O. The role of hyperbaric oxygen therapy (hot) as an otoprotection agent against Cisplatin ototoxicity. Acta Cir Bras. 2008;23(Suppl 1):72–76; discussion 6
- Corradini SG, Elisei W, De Marco R, et al. Preharvest donor hyperoxia predicts good early graft function and longer graft survival after liver transplantation. Liver Transpl. 2005;11(2):140–151