Abstract
Blood gas analyses are needed to reveal any kind of acid-base imbalance in some patients. Traditionally, arterial punctures are performed to obtain the blood samples for blood gas analyses. Arterial puncture is not a completely safe procedure. It may cause serious problems including arterial thrombosis, arteriovenous fistula, pseudoaneurysms and hematoma. In this retrospective reviewing, it was aimed to yield novel formulations to predict the blood pH only from CtCO2 and HCO3 values which can easily be measured in venous blood samples obtained for other diagnostic and follow-up purposes.
Dear Editor,
Blood gas analyses are needed to reveal any kind of acid–base imbalance in some patients. Traditionally, arterial punctures are performed to obtain the blood samples for blood gas analyses. Arterial puncture is not a completely safe procedure. It may cause serious problems including arterial thrombosis, arteriovenous fistula, pseudoaneurysms, and hematoma.Citation1,Citation2 Venipuncture is the most common invasive medical procedure performed by health care providers.Citation3 Although serious complications can occur as a result of venipuncture, it is considered to be reasonably safe.Citation3 It has been also reported that blood gas analyses to determine the acid–base status may be performed in venous blood samples and venous pH, bicarbonate, and base excess are in accordance with those of arterial blood samples in various patient populations.Citation4,Citation5 However, the agreement of venous pCO2 values with arterial pCO2 values seems to be in conflict.Citation4,Citation5 In this retrospective reviewing, it was aimed to yield novel formulations to predict the blood pH only from CtCO2 and HCO3 values which can easily be measured in venous blood samples obtained for other diagnostic and follow-up purposes.
For this aim, having the local ethic committee approval, we reviewed 21,586 blood gas analyses. There were repeat analyses from the same patient at different times. Venous or arterial sample differentiation of the blood samples was not taken into consideration due to ignoring pO2 values during the data collection. Blood gas analyses were collected from blood gas analyzers in raw digital format. SPSS 15.0 (Chicago, IL) statistics program tools were used to derive the formulations and graphic.
Total CO2 content (tCO2) in human blood is the sum of the HCO3 and pCO2. Carbamates are also taken into account [Eq. (1)].
As it can be seen, neglecting the contribution of carbamates,Citation6 pCO2 can be predicted from this equation easily if tCO2 and HCO3 concentrations are measured directly.Citation7,Citation8 Then, pH may also be predicted according to the Henderson–Hasselbalch formula [Eq. (2)].
In reviewing our data consisting of 21,586 blood gas analyses, the exploration for pCO2 estimation was gathered from this large number of arterial real blood gas reports through regression analysis aiming to reach estimated pCO2 (pCO2e) levels from total carbon dioxide content and serum bicarbonate. The ultimate formula was as follows [Eq. (3)].
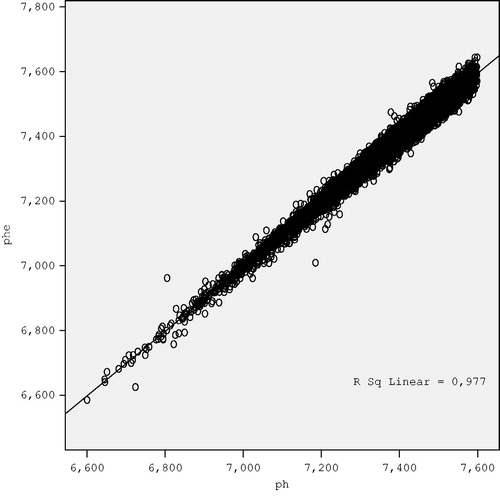
Then, estimated pH (pHe) values were calculated and they were controlled back to obtain the power of the estimation and it was seen that there was a very strong correlation between the real pH and the pHe values (r = 0.988, p < 0.001) ().
Figure 1. The chart showing the association between the real (pH) and estimated pH levels (pHe) from the equation derived from the regression analysis.
According to this method, one can easily predict blood pH from total carbon dioxide and blood bicarbonate measurements in venous blood samples obtained for other biochemical analyses avoiding the risks of arterial puncture and without need of blood gas devices. This approach may facilitate patient follow-up. However, studies which will investigate this approach in venous blood samples are necessary. Additionally, this study and formulation may at least help for in vitro or animal studies aiming to use associations between blood bicarbonate, total carbon dioxide content, and pH levels.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bergentz SE, Hansson LO, Norbäck B. Surgical management of complications to arterial puncture. Annals of surgery. 1966;164(6):1021–1026
- Bobbia X, Grandpierre RG, Claret P-G, et al. Ultrasound guidance for radial arterial puncture: a randomized controlled trial. Am J Emerg Med. 2013;31(5):810–815
- Galena HJ. Complications occurring from diagnostic venipuncture. J Fam Prac. 1992;34(5):582–584
- Ak A, Ogun CO, Bayir A, Kayis SA, Koylu R. Prediction of arterial blood gas values from venous blood gas values in patients with acute exacerbation of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2006;210(4):285–290
- Kelly A-M. Review article: can venous blood gas analysis replace arterial in emergency medical care. Emerg Med Australas. 2010; 22(6):493–498
- Arthurs G, Sudhakar M. Carbon dioxide transport. Contin Educ Anaesth Crit Care Pain. 2005;5(6):207–210
- Kobold U, Dülffer T, Dangl M, et al. Comparison of measured and calculated bicarbonate values. Clinical Chemist. 2008;54(9):1586–1587
- Chittamma A, Vanavanan S. Comparative study of calculated and measured total carbon dioxide. Clin Chem Lab Med. 2008;46(1):15–17
