Abstract
Tubular intraluminal inflammatory cells may be seen in kidney biopsies of patients with pyelonephritis, cell-mediated transplant rejection, autoimmune tubulointerstitial nephritis, allergic reactions, or in association with monoclonal light chain casts. When casts in a native kidney are primarily composed of granulocytes, the cause is most commonly acute pyelonephritis due to an ascending bacterial urinary tract infection. We report a 57-year-old man with acute kidney injury and an intense intraluminal neutrophil response to monoclonal lambda light chain crystal containing casts.
Background
Multiple myeloma and other lymphoproliferative disorders often cause kidney disease. The spectrum of associated kidney pathology includes myeloma kidney (cast nephropathy), amyloidosis, light chain proximal tubulopathy (LCPT), monoclonal immunoglobulin deposition disease, and rarely monoclonal light chain deposits along the tubular basement membranes. Tubular intraluminal inflammatory cells may be seen in kidney biopsies of patients with monoclonal light chain casts, but may also be seen in pyelonephritis, cell-mediated transplant rejection, autoimmune tubulointerstitial nephritis, or allergic reactions.1 When the primary composition of the inflammatory component are granulocytes, acute pyelonephritis is the most common finding.2 We present an interesting biopsy finding in a patient with multiple myeloma who presented with acute kidney failure. The histology revealed an intense neutrophilic inflammatory response associated with crystallization of light chains of lambda (λ) type in the lumen of tubules.
Case report
Clinical history and initial laboratory data
A 57-year-old African-American man presented to the hospital with weakness and fatigue of 2 weeks duration. He had not sought medical care for many years and was not currently taking any medications. On admission, the serum creatinine level was 7.4 mg/dL. On examination, the patient appeared ill with a weight of 193 pounds and a body mass index of 26.2 kg/m2. His heart rate was 70 beats/min and regular and blood pressure was 130/80 mmHg. Examination of chest and abdomen were unremarkable. Extremities showed no edema. Laboratory findings at presentation are listed in , notable for urinalysis with dipstick trace positive for albumin. The spot urine protein/creatinine ratio was 1.2. He had a leukocytosis with neutrophilia (86% neutrophils) but no immature band forms seen. Anemia was also discovered on presentation. The kidneys were markedly enlarged by renal ultrasonogram, with long axis dimensions measuring 16.3 cm for the right kidney and 15 cm for the left kidney. Renal cortical echogenicity was normal, and there was no evidence of a mass, stone or hydronephrosis. A kidney biopsy was arranged before results of serologic testing were available to determine the etiology of kidney injury.
Table 1. Laboratory results.
Kidney biopsy
Tissue submitted for light microscopy contained four (4) cores of renal cortex containing sixty-two (62) glomeruli, seven (7) of which were globally sclerosed. The remaining glomeruli were relatively unremarkable without cellular proliferation, capillary wall changes or expansion of mesangium. The distal tubules showed marked, diffuse white blood cell casts composed of mainly neutrophils, which were also infiltrating the tubular epithelium (). There were strongly eosinophilic, sharply demarcated crystalline (mostly rhomboid, rectangular, and needle shaped) structures of varied geometric shapes within tubular lumina admixed focally with the neutrophil casts (). The tubular lumina contained exclusively crystalline structures without fragmented amorphous proteinaceous casts, having a variegated staining pattern. These crystal containing casts differ from Bence–Jones cast nephropathy as well as other forms of tubulointerstitial injury seen in the setting of plasma cell dyscrasia (). Several tubules were ruptured with a surrounding non-caseating granulomatous reaction. There was marked interstitial edema as well as diffuse acute inflammation composed of neutrophils and eosinophils. The proximal tubules were spared though they appeared relatively simplified. The arterial vessels did not show significant sclerosis or display an accumulation of hyaline or amorphous pale-staining material. A Congo red stain was negative in the glomeruli, tubulointerstitium, and vessels.
Figure 1. (A) Tubules showing extensive neutrophil inflammation with eosinophilic crystals (A. 20× [inset 60×], H&E). (B) Granulomatous response to tubular rupture (20×, H&E). (C) Rhomboid and needle shaped intraluminal crystals (40×, H&E). (D) Ultrastructurally, there were needle shaped and rhomboid intraluminal hyperdense crystalline structures (×4000).
![Figure 1. (A) Tubules showing extensive neutrophil inflammation with eosinophilic crystals (A. 20× [inset 60×], H&E). (B) Granulomatous response to tubular rupture (20×, H&E). (C) Rhomboid and needle shaped intraluminal crystals (40×, H&E). (D) Ultrastructurally, there were needle shaped and rhomboid intraluminal hyperdense crystalline structures (×4000).](/cms/asset/60dbf0a4-bb25-4848-a632-f1fc96105730/irnf_a_844643_f0001_b.jpg)
Table 2. Tubulointerstitial diseases associated with plasma cell dyscrasias.
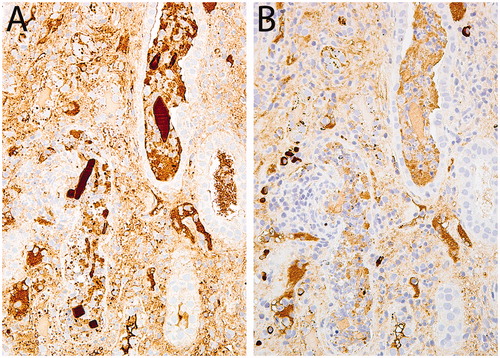
Immunofluorescence (IF) microscopy (renal cortex with 14 glomeruli) revealed no glomerular staining for IgG, IgA, lgM, C3, C1q, kappa, or lambda light chains. The tubulointerstitial compartment was also negative for all antibodies, but no casts were identified in the frozen tissue by DiffQuik analysis. Special stains for bacteria (Gram stain), fungus (GMS), and acid fast bacilli (AFB) were all negative. Immunoperoxidase staining on the formalin-fixed paraffin embedded tissue for kappa and lambda light chains showed strong lambda positivity (3+) within the well-defined, intra-tubular crystals and no significant kappa staining ().
Figure 2. A&B: The crystals stained intensely (3+) for lambda light chains (A) but not (0) for kappa light chains (B), Immunoperoxidase stains, 40×.
Electron microscopy showed several tubular segments containing rectangular or rhomboid-shaped, osmiophilic crystals in the lumina with well defined, sharp edges, surrounded by predominantly neutrophils and admixed with the crystalline deposits (). The tubular epithelial cells demonstrated marked injury but no intraepithelial crystals were present in a large sample examined. On EM of the glomerulus, the capillary basement membranes display a smooth and regular contour and a mean thickness of 400 nm. The foot processes were preserved and the visceral epithelial cells were unremarkable. No immune complex-type electron dense deposits, fibrillar, or crystalline deposits were seen along the capillary basement membranes, in glomerular cells or within the mesangium.
Diagnosis
Bence–Jones (monoclonal lambda light chain) crystal containing cast nephropathy with a predominant granulocytic response.
Clinical follow-up
Following the biopsy results, the patient was found to have a monoclonal IgG-lambda protein measuring 2.8 g/dL by serum protein electrophoresis and immunofixation studies along with elevated serum free lambda light chains (5140 mg/L) and markedly decreased free kappa/Lambda ratio (0.01). A bone marrow biopsy showed 19% monoclonal plasma cell of λ-type. A bone survey showed superficial intracortical osteolytic lesion in the anterior aspect of the right femoral shaft. The patient had fever of unknown origin, which delayed chemotherapy initiation for 2 weeks. Blood and urine cultures were repeatedly negative despite being on no antibiotic therapy for the first few days of admission and prior to the biopsy. Infectious disease specialists evaluated the patient and concluded the fever and elevated ESR were due to the underlying malignancy. His initial low albumin was ascribed to poor PO intake, inflammation, and potential chronic liver disease.
Treatment for symptomatic multiple myeloma was started with Bortezomib, receiving four courses (1.3 mg/m2/day, four infusions at Days 1, 4, 8, and 11) with dexamethasone. The kidney failure resolved after three cycles of therapy, and patient no longer required hemodialysis after four weeks of chemotherapy. After treatment, the eGFR and creatinine were >60 mL/min and 1.0 g/dL respectively, 30 days following start of chemotherapy. Hemoglobin increased to 12.4 g/dL and the WBC count decreased to 9.9 × 103/L. The serum-free lambda light chain decreased to 70.11 mg/L (Kappa/Lambda ratio 0.12 L). Currently, the patient is being evaluated for an autologous stem cell transplant.
Discussion
Renal injury in a patient with monoclonal light chains is most commonly due to Bence–Jones cast nephropathy (myeloma kidney), monoclonal immunoglobulin deposition disease (MIDD), amyloidosis, or light chain proximal tubulopathy (LCPT).Citation3 In LCPT the excessive light chains (LCs), mostly kappa type, are excreted through the kidney and are reabsorbed in the proximal tubular cells with or without crystal formation in the cytoplasm, leading to tubular damage. This often results in acquired Fanconi syndrome characterized by aminoaciduria, glycosuria, phosphaturia, bicarbonate, and uric acid wasting.Citation4 When MIDD and AL amyloidosis affect the tubulointerstitial compartment, they tend to deposit along the tubular basement membranes leading to irregular thickening either with PAS positive material which stains for the monoclonal light chain and leads to acute renal failure (MIDD) or an amorphous waxy accumulation of PAS negative, Congo Red positive material frequently in a globular pattern (amyloid).Citation3 Rarely amyloid casts may also precipitate within the tubular lumen, also congo red positive.Citation5 Cast nephropathy shows precipitation of sharply-demarcated, brittle casts admixed with uromodulin in the distal tubules with a distinct morphologic and staining pattern. Monoclonal light chains may also deposit within the tubular basement membranes and cause non-specific tubulointerstitial nephritis, wherein the deposits may only be localized using immunoelectron microscopic methods, called immunogold labeling.Citation6
This case is unique in that the intraluminal casts are more crystalline in appearance and elicit a striking neutrophil-rich response which mimics acute pyelonephritis. Acute pyelonephritis was excluded by serially negative urine cultures and lack of bacterial organisms within the biopsy, a common finding in pyelonephritis.Citation2 Anecdotally, this response has been reported in reference textsCitation7 but to our knowledge is not present in the primary literature. Other types of plasma cell related injury can be excluded by the presence of normal glomeruli and lack of tubular basement membrane thickening (MIDD and amyloid). No tubular intraepithelial cell crystal accumulation or increased lysosomal bodies were present excluding proximal tubulopathy. However, brightly eosinophilic, sharply demarcated crystalline structures of varied geometric shapes were focally present intermixed with abundant neutrophils in the distal tubular lumina, which stained exclusively for λ light chains, diagnostic of monoclonal lambda crystal deposits.
Both lambda and kappa light chains are normally filtered through the glomeruli, reabsorbed, and metabolized in proximal tubules. When present in excess, such as in plasma cell dyscrasias, the filtered load of abnormal light chains overwhelms the proximal tubular reabsorption capacity, potentially leading to a downstream precipitation as amorphous casts with tubular protein (Bence–Jones cast nephropathy) or crystallization.Citation3 Cast nephropathy typically develops when the abnormal monoclonal light chains have an affinity for binding to Tamm–Horsfall protein (uromodulin) and precipitate, leading to obstruction of the distal convoluted tubules.Citation8 In this case, interestingly, the crystalline material largely does not have incorporated Tamm–Horsfall protein. Previously described by Sethi et al.,Citation5 pathologic casts in the setting of multiple myeloma may be myeloma (Bence–Jones) protein casts, light chain crystal casts, or rarely light chain amyloid casts.
Inflammation may be seen in association with light chain (Bence–Jones) casts in the distal tubules, but when present is usually composed of mononuclear cells and multinucleated foreign body giant cells. The inflammatory infiltrate may also involve the interstitium. In a typical case, the intraluminal inflammation is believed to develop from direct transmigration of inflammatory cells through the basement membrane into the tubular lumen.Citation9,Citation10
In the setting of multiple myeloma or other plasma cell dyscrasias, the abnormal monoclonal light chains may have unique conformational changes within the amino acid structure, which are believed to induce specific pathological changes in specific organs (heart, liver, kidney).Citation11,Citation12 Taking the abnormal light chains from a patient with cast nephropathy and injecting them into rats causes cast nephropathy in the rat kidneys, and similarly for amyloidosis and MIDD.Citation11 Specific amino acid mutations have been identified that makes certain light chains more likely to be pathogenic to tissues causing disease, particularly in the light chain variable domain region.Citation13–15 Under appropriate intraluminal conditions of pH, concentration, and flow rates, they may crystallize and are often resistant to proteolysis within cells. We speculate that, in the absence of active pyelonephritis, the neutrophil rich tubular response has developed because of the chemotactic properties of the unique monoclonal lambda light chain crystals within the renal tubules in this patient.
Light chains are known to have an effect on neutrophils under normal and disease-associated conditions. Polyclonal as well as monoclonal light chains, when tested in vitro, have been shown to inhibit neutrophil apoptosis and migration.Citation16 Tamm–Horsfall protein, which is also involved in the cast nephropathy process, may also play a key role by activating granulocytes.Citation17 In conjunction, one or both of these processes may contribute to an increased number and altered distribution of neutrophils when exposed to significant amounts of abnormal light chains under the appropriate conditions.
A neutrophil-rich reaction to monoclonal Bence–Jones proteins, in particular the predominantly tubular intraluminal crystalline deposits, has been rarely mentioned in the literature.Citation7 The identification and distinction between acute pyelonephritis and crystal storing nephropathy with chemo-attractive properties is significant because of major hematologic work-up, treatment, and prognostic implications, in middle and older age patients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bonsib SM, Bergstein J. ARF with casts eliciting a cellular reaction. Am J Kidney Dis. 2004;43(6):1138–1141
- Ivanyi B, Rumpelt HJ, Thoenes W. Acute human pyelonephritis: leukocytic infiltration of tubules and localization of bacteria. Virchows Archiv A Patholog Anatomy Histopathol. 1988;414(1):29–37
- Herrera GA PM. Renal Diseases Associated with Plasma Cell Dyscrasias, Amyloidosis, Waldenstrom Macroglobulinemia, and Cryoglobulinemic Nephropathies. Vol. 2, 6th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2007
- Larsen CP, Bell JM, Harris AA, Messias NC, Wang YH, Walker PD. The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Modern Pathol. 2011;24(11):1462–1469
- Sethi S, Hanna MH, Fervenza FC. Unusual casts in a case of multiple myeloma. Am J Kidney Dis. 2009;54(5):970–974
- Gu X, Herrera GA. Light-chain-mediated acute tubular interstitial nephritis: a poorly recognized pattern of renal disease in patients with plasma cell dyscrasia. Arch Pathol Lab Med. 2006;130(2):165–169
- Seshan SV, D'Agati VD, Appel GA, Churg J, eds. Renal disease classification and Atlas of tubulo-interstitial and vascular diseases. In Tubulo-interstitial and Vascular Lesions Associated with Neoplastic Disorders. Baltimore, MD: Williams and Wilkins; 1999
- Huang ZQ, Kirk KA, Connelly KG, Sanders PW. Bence Jones proteins bind to a common peptide segment of Tamm-Horsfall glycoprotein to promote heterotypic aggregation. J Clin Invest. 1993;92(6):2975–2983
- Cohen AH, Border WA. Myeloma kidney. An immunomorphogenetic study of renal biopsies. Lab Invest A J Tech Methods Pathol. 1980;42(2):248–256
- Sedmak DD, Tubbs RR. The macrophagic origin of multinucleated giant cells in myeloma kidney: an immunohistologic study. Human Pathol. 1987;18(3):304–306
- Solomon A, Weiss DT, Kattine AA. Nephrotoxic potential of Bence Jones proteins. New Engl J Med. 1991;324(26):1845–1851
- Comenzo RL, Zhang Y, Martinez C, Osman K, Herrera GA. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98(3):714–720
- Aucouturier P, Bauwens M, Khamlichi AA, et al. Monoclonal Ig L chain and L chain V domain fragment crystallization in myeloma-associated Fanconi's syndrome. J Immunol. 1993;150(8 Pt 1):3561–3568
- Deret S, Chomilier J, Huang DB, Preud'homme JL, Stevens FJ, Aucouturier P. Molecular modeling of immunoglobulin light chains implicates hydrophobic residues in non-amyloid light chain deposition disease. Protein Eng. 1997;10(10):1191–1197
- Abraham RS, Geyer SM, Price-Troska TL, et al. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL). Blood. 2003;101(10):3801–3808
- Cohen G, Horl WH. Free immunoglobulin light chains as a risk factor in renal and extrarenal complications. Semin Dial. 2009;22(4):369–372
- Kreft B, Jabs WJ, Laskay T, et al. Polarized expression of Tamm-Horsfall protein by renal tubular epithelial cells activates human granulocytes. Infect Immun. 2002;70(5):2650–2656
