Abstract
Background: There is a paucity of research on platelet apoptosis and its contribution to platelet dysfunction in uremic patients. The present study sought to analyze platelets apoptosis in uremic patients who underwent different dialysis modalities. Methods: Sixteen chronic uremic patients (5 on hemodialysis, 6 on peritoneal dialysis and 5 on non-dialysis) and 16 controls were studied. Platelet-rich plasma was detected for apoptotic events including depolarization of mitochondrial inner membrane potential (ΔΨm), phosphatidylserine (PS) exposure, activation of caspases-3 and Bcl-2 family proteins variations by Flow Cytometry or by Western-Blot. Washed normal platelets were incubated with normal or uremic platelet poor plasma and then were detected apoptotic events. Platelets function was assessed by ristocetin induced aggregative function test. Results: Compared to controls, uremic platelets demonstrated greater apoptosis for the ΔΨm depolarization (43.48 ± 9.58 vs. 52.76 ± 15.36, p = 0.005) as well as PS exposure (1.36 ± 0.51 vs. 0.99 ± 0.27, p < 0.001). There was no significant difference among different treatment groups (for the ΔΨm depolarization f = 0.16, p = 0.85; for the PS exposure f = 1.06, p = 0.36). Western Blot analyses showed caspase-3 activation and pro-apoptotic Bcl-2 family proteins expression. Platelets exposed to uremic plasma exhibited distinct apoptosis phenomena. Ristocetin induced platelet aggregation was markedly diminished in uremic patients and treated platelets. Conclusions: These findings indicate that platelets are incurred apoptosis in uremia patients. Uremic plasma accelerates apoptosis of normal platelets, resulting in a dysfunctional pattern of platelets in uremia. Uremic platelets apoptosis has no relationship with dialysis modality.
Introduction
Apoptosis is a highly regulated, normal physiological process of programmed cell death. Abnormal regulation in excessive or reduced amounts of cells has pathological implications.Citation1,Citation2 For many years, apoptosis is attributed exclusively to nucleated cells. However, recent studies show that anucleated platelets are also capable of performing apoptosis.Citation3–5 Diverse cell-external chemicalCitation6,Citation7 and physical stimuliCitation8,Citation9 can induce transformation of resting platelets to an apoptotic state. Generally, apoptotic events include the mitochondrial inner transmembrane potential (ΔΨm) depolarization, expression changes of Bcl-2 protein family, cytochrome c release from mitochondria to the cytosol, activation of caspases, cleavage of cytoskeleton proteins, exposure of acidic aminophospholipid phosphatidylserine (PS) on the platelet plasma membrane, platelet shrinkage, shedding of platelet-derived microparticle and blebbing and filopod formation on platelet membrane.Citation10–12
Bleeding tendency of uremic patients, from mild bruising to more severe gastrointestinal or intracranial hemorrhage,Citation13 has been attributed classically to abnormalities of platelets function that include impaired adhesion and decreased aggregation. The pathogenesis of the platelet dysfunction is multifactorial and includes intrinsic platelet defects, anemia, uremic toxins, von Willebrand factor and vessel abnormalities.Citation14 Among them, there is a strict interaction between uremic toxins and the biologic system. Toxins can trigger a number of cellular responses such as phagocytosis and apoptosis.Citation15 Studies have already shown that nucleated cells, such as monocytes, neutrophils and erythrocytes obtained from uremic subjects, undergo accelerated apoptosis.Citation16,Citation17 As an anucleated cell, however, little is known of platelet apoptosis in uremia or its contribution to platelet dysfunction. Consequently, the aim of the present study was to investigate the contribution of apoptosis to platelet dysfunction in uremic patients. This was achieved by examining whether uremic patients undergo accelerated apoptosis and whether uremic plasma influences platelets apoptosis and functions.
Materials and methods
Study design
Platelet-rich plasma (PRP) from three groups of uremic subjects, i.e., hemodialysis (HD) patients, peritoneal dialysis (PD) patients, non-dialysis patients (ND) and matched healthy volunteers were processed for quantification of apoptosis. To further insure platelet apoptosis and to evaluate the impact of uremic plasma on platelets functions, platelets from healthy volunteers were incubated in culture medium supplemented with “normal” or “uremic” platelet-poor plasma (PPP), respectively. After the incubation period, platelets aliquots were processed for quantification of apoptosis and function test. Every experiment was repeated once time to reduce deviation and was performed in parallel with negative and positive control.
Patients
The principal characteristics of the uremic patients are presented in . We studied 16 patients, of them, 5 on HD, 6 on PD, and 5 on ND, and 16 healthy controls. The uremic patients were from nephrology department of 1st Affiliated Hospital of Soochow University. The controls, 16 age and gender matched healthy subjects with normal hematocrit and renal function (serum creatinine <1.5 mg/dL), were chosen from health center of the hospital.
Table 1. Patient characteristics.
Five uremic patients on chronic maintenance HD for more than 6 months were placed in the first group. The HD procedure was the same for all patients. They underwent thrice weekly dialysis of 4-hours duration via arteriovenous fistula using cellulose triacetate hollow-fiber dialyzers and heparin anticoagulation.
Six uremic patients on chronic PD modality were placed in the second group. They were dialyzed with continuous ambulatory peritoneal dialysis (CAPD, Tenckhoff catheter) using the Baxter Twin Bag (1500–2500 mL/time, 3–4 times/d, depending on body size).
The third, ND group, included five subjects with advanced chronic renal impairment (creatinine clearance <15 mL/min) not on dialysis. Of the drugs taken by HD, PD and ND patients, phosphate binders, calcitriol, cholecalciferol, iron and folate were especially prominent. Antihypertensive therapies included calcium blockers, β-blockers and angiotensin-converting enzyme inhibitors.
The exclusion criteria included (i) acute infection or blood transfusion in the past 3 months; (ii) unstable clinical conditions including vascular and cardiac instability; (iii) a positive history of diabetes mellitus, malignancy, immunological disease, nephrotic syndrome or have a pre-existing hemostatic disorder unrelated to uremia. All of the patients had been free of medications known to affect hemostasis (except erythropoietin) for at least 1 month prior to the study. Collection of blood samples was approved by the Human Investigation Review Committee, and all participants gave informed consent.
Reagents and equipments
Tetramethylrhodamine ethyl ester (TMRE) was from Bender Medsystem (Vienna, Australia). FITC anti-human CD62P and purified anti-Caspase-3 were purchased from Biolegend (San Diego, CA). FITC-conjugated Annexin V was from Beyotime Biotechnology (Haimen, China). FITC-conjugated Goat Anti-Mouse IgG was from Bioword Technology (Minneapolis, MN). HRP-conjugated goat anti-mouse IgG and monoclonal antibodies against Bax, Bcl-xL, Bcl-2 were from Santa Cruz Biotechnology (Paso Robles, CA). Automatic biochemical analyzer 7100 was from HITACHI (Tokyo, Japan). Turbidimetric platelet aggregometer was from Chrono-Log Corporation (Havertown, PA).
Hemorrhagic symptoms
Hemorrhagic symptoms occurring during the 30 days before the investigation were defined as current symptoms and were collected in the three groups and controls. They included epistaxis, gingival and genital bleeding, petechiae, ecchymosis, hematuria, hemoptysis, hemarthrosis and telangiectasis.
Blood sampling
Fresh blood from subjects were anti-coagulated with 1/7 volume of acid-citrate dextrose (ACD, 2.5% trisodium citrate, 2.0% d-glucose, 1.5% citric acid). After centrifugation, isolated platelets were washed twice with CGS buffer (0.123 M NaCl, 0.033 M d-glucose, 0.013 M trisodium citrate, pH 6.5) and resuspended in modified Tyrode's buffer (2.5 mM Hepes, 150 mM NaCl, 2.5 mM KCl, 12 mM NaHCO3, 1 mM CaCl2, 1 mM MgCl2, 5.5 mM d-glucose, pH 7.4) to a final concentration of 3 × 108 /mL. In PRP experiments, fresh blood was anti-coagulated with 1/9 volume of 3.8% trisodium citrate, and centrifuged at 150g for 12 min to get PRP. Then, washed platelets and PRP were incubated at room temperature (RT) for 1 h to recover to resting state as described previously. PPP was obtained by centrifugation of whole blood at room temperature for 10 min and 20 min respectively at 2500g. The supernatant was stored in −20 °C for further experiments. To evaluate the effect of uremia on platelets apoptosis, washed platelets from normal controls were pre-incubated with PPP at 37 °C for 3 h, and then were detected. Subjects on chronic HD treatment had blood collected from a functioning fistula just prior to dialysis after the long interdialytic interval.
Platelet apoptosis evaluation
ΔΨm depolarization measurement
ΔΨm was determined using the potential sensitive dye TMRE. Briefly, PRP (3 × 108 /mL) were incubated with TMRE (100 nM) in the dark at 37 °C, 90 rpm for 20 min, then was followed by flow cytometry analysis. TMRE signals were excited using a 488 nm krypton-argon laser line and captured using filters at 625 nm.
Bcl-2 protein family expression
The sample was lysed with an equal volume of lysis buffer containing 0.1 mM E64, 1 mM phenylmethyl sulfonyl fluoride (PMSF) and 1/100 aprotinin on ice for 30 min. The whole lysate was resolved by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western analyses were performed using the following conditions: Bax (1:500), Bcl-2 (1:500), or Bcl-XL (1:500), respectively. GAPDH (1:10000) antibody was used to confirm that protein inputs in each lane were similar.
Determination of caspase 3 activity
The sample was assayed for caspase 3 activation by Western Blot analyses using caspase 3(1:1000).
PS exposure assay
PS was detected by flow cytometry using FITC-conjugated annexin V probe. The sample was mixed with the Annexin V binding buffer and annexin V-FITC at a 50:10:1 ratio, then were gently mixed by rocking and incubated at RT for 20 min in the dark. PS exposure was detected by flow cytometry then.
Platelet activation detection
The sample was incubated with FITC-antihuman CD62P (p selectin, 10:1) at RT in the dark for 20 min, then was subjected to flow cytometry analysis.
Platelets aggregation function tests
For aggregation studies, ristocetin (1.25 mg/mL) induced aggregative ability of the sample was measured by a turbidimetric platelet aggregometer at 37 °C with a stirring speed of 1 krpm. The tests for dialysis patients were performed on an off-dialysis day to avoid any confounding effects of heparin exposure during dialysis.
Statistical analysis
Data are presented as mean ± SD. Statistical analysis was performed using the SPSS 11.5 software package (SPSS CHINA, Shanghai, China). A p value < 0.05 was considered statistically significant. Intra-group comparison was made using the paired Student's t-test. Quantitative clinical data was compared between two groups by Fisher exact test. Multiple groups were analyzed by analysis of variance (ANOVA). Univariate analysis was performed using Pearson's correlation.
Results
Patient characteristics
With respect to laboratory values, the uremic subjects had relatively lower platelet counts, and as expected, had greater serum creatinine (SCr) concentrations (). There was no significant difference among HD, PD and ND group for the parameter of platelets count and SCr concentrations (p > 0.05). The etiology of renal failure was chronic glomerulonephritis (n = 7), nephroangiosclerosis (n = 6) and chronic interstitial nephritis (n = 3).
Assessment of hemorrhagic symptoms
Hemorrhagic symptoms which included ecchymosis, gingival and genital bleeding, epistaxis, petechiae and telangiectasis, were found in 6 uremic patients (37.50%) and in 1 control (6.25%). The percentage of subjects with hemorrhagic symptoms was significantly higher in uremic groups than in controls (p = 0.04).
Detection of apoptosis in uremic platelets
Compared with controls, ΔΨm depolarization, as monitored by flow cytometry analysis with decreased fluorescence intensity in TMRE-stained platelets, was significantly obvious in uremic patients (, ). There was no statistical significance among HD, PD and ND groups (f = 0.16, p = 0.85).
Figure 1. Platelets apoptosis of 16 uremic patients. ΔΨm depolarization, as monitored by flow cytometry analysis with decreased fluorescence intensity in TMRE-stained platelets, was significantly obvious in uremic patients. HD, PD and ND groups had significant differences from controls. (A). Uremic platelets had enhanced FITC fluorescence intensity which represented PS exposure. HD, PD and ND groups had significant differences from controls. (B) For Western-Blot assay, a 17 kD fragment which exhibited caspase-3 activation was emerged in uremic platelets. (C) The expression changes of Bcl-2 protein family showed obviously decreased levels of Bcl-xL and Bcl-2, and enhanced level of Bax. (D) *p < 0.05, **p < 0.001.
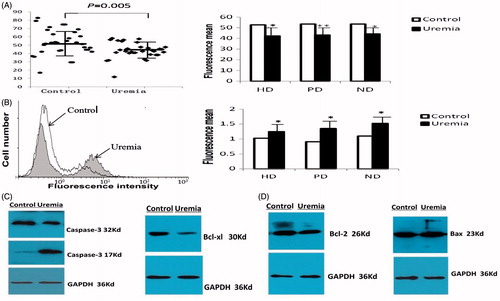
Table. 2. ΔΨm, PS exposure and P-selectin expression in three groups of uremic patients.
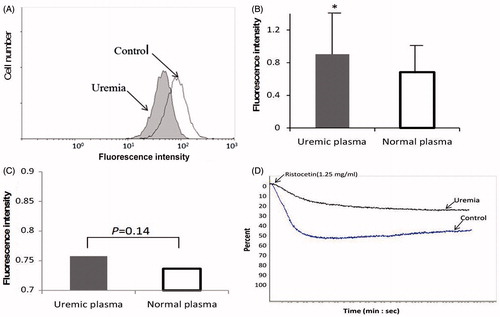
As shown in , , expression of PS on the platelet surface was significantly increased in all of three groups compared to controls. There was no statistical significance among HD, PD and ND groups (f = 1.06, p = 0.36).
Activated caspases initiate, propagate and amplify apoptotic signaling. As shown in , a 17 kD fragment which exhibited caspase-3 activation was emerged in uremic platelets. Likewise, the expression level of Bax was significantly enhanced; however, obviously decreased expression level was observed in Bcl-2 and Bcl-XL ().
Effect of uremic plasma on apoptosis of normal platelets
Platelets from healthy volunteers incubated with heterologous uremic PPP were next used as a model to study the effect of uremic plasma on apoptosis of normal platelets. Eight uremic patients (3ND, 3PD, 2HD) were chosen randomly for preparing uremic PPP. Compared with platelets from healthy volunteers exposed to heterologous normal plasma, those exposed to uremic plasma exhibited higher apoptosis phenomenon: for ΔΨm depolarization (48.99 ± 15.34 vs. Control 69.96 ± 29.32, p = 0.0001); for PS exposure (0.91 ± 0.54 vs. Control 0.68 ± 0.37, p = 0.002) (). In treated platelets, the expression level of Bax was significantly enhanced; Bcl-2 and Bcl-XL were obviously decreased. Caspase-3 activation was emerged in pre-conditioned platelets.
Detection of platelet activation
The surface expression of P-selectin was analyzed with flow cytometry. The results showed that there was no obvious statistic difference between ND, PD, HD and control (). After incubated with uremic plasma, normal platelets didn't show obvious surface expression of P-selectin (0.76 ± 0.21 vs. Control 0.74 ± 0.20, p = 0.14, ).
Platelets aggregation function
As shown in , ristocetin-induced platelet aggregation was markedly diminished in uremic patients and uremic-PPP-incubated platelets.
Determinants of platelets apoptosis
In uremic patients, we analyzed the relationship between platelets apoptosis and creatinine clearance rate. Creatinine clearance rate negatively correlated with PS exposure (r = −0.18) and positively correlated with ΔΨm depolarization (r = 0.18) but without significance (p = 0.38, p = 0.32, respectively).
Discussion
The aim of this study was to assess platelet apoptosis in uremic patients and to investigate the effect of uremic plasma on platelet apoptosis. The main findings were that (i) several uremic patients, no matter which kind of dialysis method they accepted, had bleeding symptoms; (ii) markers for apoptosis, including ΔΨm depolarization, Bcl-2 protein family expression changes, Caspase-3 activation and PS exposure, exhibited an enhanced platelet apoptosis in uremic patients; (iii) platelets from HD, PD and ND patients had similar apoptosis manifestations; (iv) uremic plasma induced enhanced apoptosis and aggregation dysfunction in normal platelets; and (v) there was no significant correlation between platelet apoptosis and creatinine clearance rate.
It is widely known that patients with chronic kidney disease are at increased risk for bleeding episodes. Although various rates have been citied in uremic patients in different publications, the bleeding diathesis rate is around 10–15%, and bleeding-associated morbidity around 15%.Citation18 In this study, compared to the controls, hemorrhagic symptoms were nearly 6-fold more frequent in uremic patients whatever dialysis method they accepted. It was probably due to the small sample size limits to some extent the generalization of the findings made in the study. There is clearly a need for prospective, multicenter, large-scale trials in the future regarding the bleeding tendency of uremic patients.
Mild and obvious thrombocytopenia was found among uremic patients in this study. Although it is assumed that it may largely due to platelets dysfunction and toxic effects from urea and other retention solutes, the etiology of uremic bleeding and thrombocytopenia is biochemically complex and not completely understood. Apoptosis is an energy dependent physiological mechanism that regulates cell life span and controls cell number. The effect of apoptosis on platelets dysfunction is still unknown. Since platelets do not contain a nucleus, some classic apoptosis markers are not applicable to revealing apoptosis. According to recommendation from literature,Citation19,Citation20 a number of key parameters of the intrinsic pathway of apoptosis had been used simultaneously in this study. Collapse of ΔΨm, which characterizes depolarization of mitochondrial inner membrane potential, is one of the major upstream markers of platelet apoptosis.Citation10 We found that ΔΨm was decreased significantly in uremic patients than in controls. This finding was confirmed by downstream markers, increased exposure of PS on the platelet plasma membrane and activation of casepase-3. Activation of caspases, which involves cleavage of inactive zymogen pro-caspase precursor, is one of the key downstream manifestations of apoptosis.Citation12 Combined with a markedly diminished platelets aggregation function, the results indicated enhanced platelets apoptosis and dysfunction in uremic patients.
Our observations are partly in accordance with report from a former research, which had shown that platelets obtained from uremic patients underwent PS exposure and caspase-3 activation, although these findings were looked as platelet activation at that time.Citation21 The process of platelet activation is associated with many specific biochemical and morphologic changes, some of which are similar to those occurring in apoptotic cells. PS exposure is assumed to be a typical event in both platelet activation and apoptosis.Citation22 However, the signaling pathways leading to PS exposure are quite different between these two processes. For the sake of distinguishing platelet apoptosis from platelet activation, P-selectin was analyzed parallelly and the data excluded the possibility of platelet activation (p = 0.14). Thus, our data support that platelet activation and apoptosis occur separately, and also indicate that during the process of platelet apoptosis, platelets are not activated obviously in this study.
This present study also demonstrates that independent of HD or PD treatment, uremia is a state that induces apoptosis. To our interesting, three groups exhibited similar platelets apoptosis events. It is generally assumed that CAPD may have advantages over such intermittent therapies as HD in regulating apoptosis and maintaining biologic function and homeostasis.Citation23 HD is a treatment itself, including the use of different dialyzers and micro-inflammation state, may influence apoptosis rate. Although there is a paucity of research on HD, PD and platelets apoptosis, the common view is somewhat conflicting with ours. This could be due to different clearance ability of HD and PD for toxins with different molecular size. While both of HD and PD were found to eliminate uremic toxins effectively and could improve hemeostasis defects in uremia, PD has the shortage of the ability to eliminate low molecular toxins.Citation24 This deficiency probably counter-balances advantages of PD. The occurrence of similar platelets apoptosis events of three groups leads one to postulate that uremic platelets apoptosis has no relationship with dialysis modality and cannot be corrected with dialysis. However, at this time the data are limited and we await further studies to delineate the issue more precisely.
Interested by the above research results, we moved forward to explore the effect of uremic toxins on platelets apoptosis and function. After incubation with uremic PPP containing numerous of retention toxins, accelerated apoptosis phenomena and function impairment were observed in preconditioned “normal” platelets. The result testified apoptosis of uremic platelets were induced by retention of uremic toxins from a different point of view. As the plasma from various uremic subjects may contain diverse molecules and demonstrates different apoptotic potential, and as the cut-off of renal function where accumulation of toxic molecules begins to affect cell life span is still unknown. It is difficult to clarify the effects of different toxins on platelets apoptosis. We suspect that enhanced uremic platelets apoptosis is induced by chronic “cooperation” of a broad spectrum of uremic toxins. Since creatinine clearance rate is generally regarded as index for renal function evaluation,Citation25 we evaluated the relationship between creatinine clearance rate and apoptosis events. The observation showed that there was no significant relationship between them. The result suggests that it is inapposite to evaluate risk of platelets apoptosis with creatinine clearance rate in end renal function stage. Considering same platelets apoptosis manifestations among HD, PD and ND groups and different capacity of three modalities to clear creatinine, we suspect that creatinine, which represents low molecular size toxin, has no influence on platelets apoptosis in uremic patients. Further study is needed in this area.
In conclusion, we demonstrate that apoptosis is accelerated in uremic platelets. Furthermore, uremic plasma induces apoptosis and dysfunction in normal platelets. It has proposed a novel pathogenic mechanism for uremic bleeding and thrombocytopenia. Meanwhile, hemodialysis, peritoneal dialysis and non-dialysis modality have similar platelets apoptosis events and this apoptosis tendency has no relationship with serum creatinine concentration. The finding is exciting and has the potential to launch further investigations with various therapeutic options portending clinical benefits.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work has been supported by Youth Scientific Fund of Soochow University (SDY2011A38).
References
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449
- Kohler C, Orrenius S, Zhivotovsky B. Evaluation of caspase activity in apoptotic cells. J Immunol Methods. 2002;265:97–110
- Kile BT. The role of the intrinsic apoptosis pathway in platelet life and death. J Thromb Hemost. 2009;7:214–217
- Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186
- Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21:871–877
- Towhid ST, Nega M, Schmidt EM, et al. Stimulation of platelet apoptosis by peptidoglycan from Staphylococcus aureus 113. Apoptosis. 2012;17:998–1008
- Leytin V, Allen DJ, Lyubimov E, Freedman J. Higher thrombin concentrations are required to induce platelet apoptosis than to induce platelet activation. Br J Hematol. 2007;136:762–764
- Leytin V, Allen DJ, Mykhaylov S, et al. Pathologic high shear stress induces apoptosis events in human platelets. Biochem Biophys Res Commun. 2004;320:303–310
- Bertino AM, Qi XQ, Li J, Xia Y, Kuter DJ. Apoptotic markers are increased in platelets stored at 37 degrees C. Transfusion. 2003;43:857–866
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519
- Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219
- Carvalho AC. Bleeding in a uremia––a clinical challenge. N Engl J Med. 1983;308:38–39
- Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–322
- Vanholder R, Argiles A, Baurmeister U, et al. Uremic toxicity: Present state of the art. Int J Artif Organs. 2001;24:695–725
- Cohen G, Raupachova J, Hörl WH. The uraemic toxin phenylacetic acid contributes to inflammation by priming polymorphonuclear leucocytes. Nephrol Dial Transplant. 2013;28:421–429
- Kramann R, Couson SK, Neuss S, et al. Uraemia disrupts the vascular niche in a 3D co-culture system of human mesenchymal stem cells and endothelial cells. Nephrol Dial Transplant. 2012;27: 2693–2702
- Davenport A, Will EJ, Davison AM. Comparison of the use of standard heparin and prostacyclin anticoagulation in spontaneous and pump-driven extracorporeal circuits in patients with combined acute renal and hepatic failure. Nephron. 1994;66:431–437
- Gyulkhandanyan AV, Mutlu A, Freedman J, et al. Markers of platelet apoptosis: methodology and applications. J Thromb Thrombolysis. 2012;33:397–411
- Zhang W, Liu J, Sun R, et al. Calpain activator dibucaine induces platelet apoptosis. Int J Mol Sci. 2011;12:2125–2137
- Bonomini M, Dottori S, Amoroso L, et al. Increased platelet phosphatidylserine exposure and caspase activation in chronic uremia. J Thromb Hemost. 2004;2:1275–1281
- Gyulkhandanyan AV, Mutlu A, Freedman J, et al. Selective triggering of platelet apoptosis, platelet activation or both. Br J Hematol. 2013;161:245–254
- Sobol AB, Kaminska M, Walczynska M, et al. Effect of uremia and hemodialysis on platelet apoptosis. Clin Appl Thromb Hemost. 2013;19:320–323
- Sardenberg C, Suassuna P, Andreoli MC, et al. Effects of uraemia and dialysis modality on polymorphonuclear cell apoptosis and function. Nephrol Dial Transplant. 2006;21:160–165
- Vatankulu MA, Murat SN, Demircelik B, et al. Effect of estimated glomerular filtration rate on periprocedural myocardial infarction in patients undergoing elective percutaneous coronary intervention. Ren Fail. 2013;35:931–935