Abstract
Background: In this present study, we aimed to investigate the association between therapeutic outcomes and vascular endothelial growth factor (VEGF) G-1154A and C-936T gene polymorphisms in patients with glomerulonephritis. Methods: Thirty-eight patients with glomerulonephritis diagnosed by renal biopsy were included to the study. All patients had proteinuria at least 1 gram (g)/day in urine analysis. At the end of a yearly therapy, patients with proteinuria less than 0.5 g/day were accepted as in complete remission and they were termed as group 1. The patients with proteinuria over 0.5 g/day were accepted as in no remission and they were termed as group 2. Results: The mean age of patients in group 1 and group 2 was 35.88 ± 13.80 years and 37.30 ± 13.89 years, respectively. There were nine (50%) male and nine (50%) female patients in group 1. In group 2, seven (35%) male and 13 (65%) female patients were present. Although VEGF G-1154A (GG) gene polymorphism was found in 55% of group 2 patients, and 22.2% of group 1 patients, but the differences did not reach statistical significance. There were no statistical differences between groups in terms of other gene polymorphisms. Namely, we obtained no statistical differences between therapeutic outcomes and gene polymorphisms. Conclusions: There is a significant difference between groups in terms of VEGF G-1154A (GG) gene polymorphism, but the minority of the patient population has led to not to reach statistical significance. So, this gene polymorphism has to be investigated in larger studies.
Introduction
Glomerulonephritis is a disease that disrupts the structure and function of glomeruli. It is one of the main reasons that lead to end-stage renal failure (ESRF). It ranks third after diabetic nephropathy and hypertension in developing chronic kidney disease in our country.
Vascular endothelial growth factor (VEGF) is a member of platelet-derived growth factor family, and synthesized in various cell types in the body. VEGF has important roles in proliferation, migration, and differentiation of endothelial cells.Citation1–6 VEGF has also a major role in occurring and maintaining the fenestrae in glomerular capillary endothelial cells.Citation7
The fenestrated endothelium in glomeruli, glomerular basement membrane, and podocytes form a physical barrier against transition of macromolecules such as albumin and the other proteins in plasma.Citation8,Citation9 Given the role of VEGF in enhancing microvascular permeability, it is estimated that VEGF may regulate glomerular permeability. There is a close relationship between the distribution of fenestrae and rise in VEGF expression. The signals derived from glomerular basement membrane and podocytes are needed for formation and flattening of the fenestrae. In the absence of VEGF-A, glomerular endothelium does not flatten enough and leads loss of fenestrae. In conclusion, proteinuria will emerge in continuation.
In various studies, it has been shown that the common 936 C/T polymorphism in the 3′ untranslated region of VEGF gene is associated with plasma levels of VEGF, and the plasma level of VEGF was significantly lower in 936T allele positive patients.Citation10 On the other hand, the presence of 1154G allele was associated with higher plasma levels of VEGF.Citation11
In this study, our aim was to investigate the presence of G-1154A and C-936T gene polymorphisms, and their correlation with response to treatment in patients with glomerulonephritis.
Subjects and methods
Sixteen male and 22 female patients were enrolled in the study. After diagnosis of glomerulonephritis, angiotensin converting enzyme inhibitor ± angiotensin receptor blocker ± antilipidemic ± antiaggregant therapy have administered to these patients conservatively. Of the patients, 31 (81.6%) had steroid plus alkylating agents or steroid plus cyclosporine in addition to conservative therapy.
Patients
The study was conducted in a prospective manner in Cumhuriyet University Medical School between January 2007 and August 2011. Thirty-eight patients with glomerulonephritis diagnosed by renal biopsy were included in the study. All patients provided their written consent for the participation in this study. The study was approved by the local Ethics Committee and was in accordance with the Declaration of Helsinki.
Each patient was followed-up during a year at 3 months intervals. All patients had proteinuria at least 1 gram (g)/day in urine analysis. At the end of a yearly therapy, patients with proteinuria less than 0.5 g/day were accepted as in complete remission and they were termed as group 1. The patients with proteinuria over 0.5 g/day were accepted as not in remission and they were termed as group 2.
VEGF G-1154A and C-936T gene polymorphisms and agarose gel electrophoresis
Briefly, VEGF gene polymorphisms were determined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method with analysis of DNA samples obtained from blood leukocytes. DNA fragments were investigated by using appropriate primers. SNP scanning was performed using MnII (Fermentas, Vilnius, Lithuania) and Hsp92II (Promega, Madison, WI) restriction enzymes for 1154 G/A and 936 C/T polymorphisms, respectively. The amplified polymorphic DNA in a volume of 12.5 µL has been treated with 33 mM Tris-acetate, 10 mM Magnesium acetate, 66 mM Potassium acetate, 0.1 mg/mL BSA (37 °C; pH:7.9) containing RE tamponade, and finally 10 U/µL of tamponade-enzyme mixture for each individual. The end product has been incubated in 37 °C for 14–16 h.
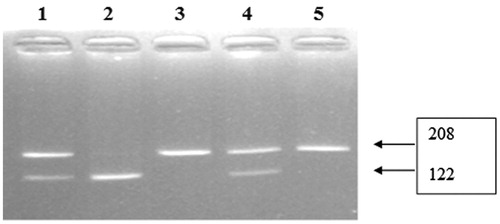
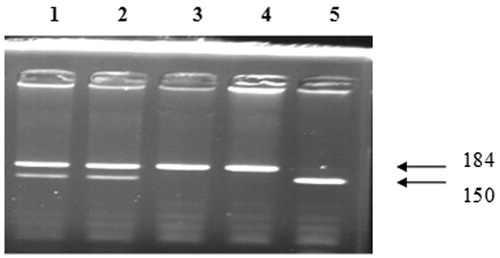
The PCR products were digested for 4 h at 37 °C with NLaIII restriction enzyme (Fermentas) at a final concentration of 5 units. After inactivation of the enzyme for 20 min at 65 °C, digested PCR products were separated by electrophoresis using a 3% agarose gel. RFLP band patterns that were visualized by ultraviolet light (Spectroline, New York, NY) are shown in and . Positive and negative controls were used to ensure genotyping accuracy.
Statistical analysis
All statistical analyses were performed using SPSS version 14.0 (SPSS, Chicago, IL). Variables following normal distribution are presented as the mean ± standard deviation. Mann–Whitney U-test was used to test for differences between independent samples. The Chi square test or Fisher's exact test were used for the comparison of polymorphisms. A p value of <0.05 was considered statistically significant.
Results
The mean age of patients in group 1 and group 2 was 35.88 ± 13.80 years and 37.30 ± 13.89 years, respectively. There were nine (50%) male and nine (50%) female patients in group 1. In group 2, seven (35%) male and 13 (65%) female patients were present. The characteristics of all patients are displayed in . The histopathological diagnoses of the patients are summarized in .
Table 1. The baseline characteristics of the patients.
Table 2. The percentage of histopathological diagnosis of the patients.
VEGF 936 CC gene polymorphism has been detected in 80% of group 2 and 72.2% of group 1. VEGF 936 CT gene polymorphism has been detected in 15% of group 2 and 16.7% of group 1. VEGF 936 TT gene polymorphism has been detected in 5% of group 2 and 11.1% of group 1. These differences were not statistically significant between group 1 and 2 ().
Table 3. The comparison groups in terms of VEGF C-936T gene polymorphisms.
VEGF 1154 AA gene polymorphism were not seen in group 2; however, it was detected in two patients of group 2 (11.1%). VEGF 1154 GA gene polymorphism was found in 45% of group 2 (n = 9) and 66.7% of group 1 (n = 12). VEGF 1154 GG gene polymorphism was detected in 55% of group 2 (n = 11) and 22.2% of group 1 (n = 4) (). There were no significant differences between group 1 and 2 in terms of VEGF 1154 gene polymorphisms. In conclusion, the differences between group 1 and 2 were not statistically significant in terms of VEGF C-936T and VEGF G-1154A gene polymorphisms.
Table 4. The comparison groups in terms of VEGF G-1154A gene polymorphisms.
Discussion
Glomerulonephritis may show variable clinical courses. Some may display sudden onset and then spontaneous recovery or treatment induced complete remissions may evolve. In contrast, some leads to rapidly progressive glomerulonephritis in a short time or chronic kidney disease in years. This process may be affected by age, gender, presence of crescent, tubulointerstitial involvement, degree of proteinuria, and ACE gene polymorphism, however, this situation is still not clear.
VEGF is synthesized by glomerular podocytes, and VEGF receptors are found on glomerular capillary endothelial cells.Citation1,Citation7,Citation12 Vascular endothelial growth factor receptor-1 (VEGFR1) and vascular endothelial growth factor receptor-2 (VEGFR2) are expressed in preglomerular, glomerular, and peritubular endothelial cells.Citation13–16 VEGF mainly activates VEGFR2. VEGF is essential for growth and proliferation of glomerular and peritubular endothelial cells.Citation1,Citation17 VEGF has an important role in occurring and maintaining the fenestrae in glomerular capillary endothelial cells.Citation7
In general, VEGF is expressed from hypoxic cells.Citation1,Citation18 The presence of hypoxy leads VEGF production by tubular cells.Citation1 The kidneys are quite sensitive to ischemia, and VEGF plays a vital role in ischemic situations. In ischemic damage, VEGF stimulates angiogenesis which is a protective mechanism for kidneys.Citation19 VEGF is needed for tubular hypertrophy and proliferation. It has been shown that glomerulosclerosis and tubulointerstitial fibrosis is associated with a loss of VEGF.Citation1 In rat studies, the pharmacological inhibition of VEGF release from podocytes has led to loss of fenestrae, endotheliosis, loss of podocytes, and proteinuria.Citation7,Citation20
Khakoo et al.Citation21 have stated that proteinuria leads to an increase in VEGF expression. Ayerder et al.Citation22 have shown that VEGF expression is diminished significantly when blood pressure was normalized by angiotensin converting enzyme inhibitors. Honkanen et al.Citation23 have stated that urinary VEGF level may be used as a marker in activation of membranous glomerulonephritis. In another study, urinary VEGF levels were compared between patients with nephrotic syndrome and healthy subjects, and it has been found that VEGF levels are increased in patients with nephrotic syndrome and are correlated with proteinuria.Citation24–28 Shimizu et al.Citation29 demonstrated that VEGF 165 therapy resulted in an improvement of crescentic and necrotizing lesions, endothelial cell proliferation, capillary reparation, and loss of proteinuria in rats with anti-glomerular basement membrane glomerulonephritis.
In the literature, multiple polymorphisms of VEGF gene were identified. The first is determined by Watson et al.Citation3 Some of these polymorphisms are found correlated with differences in VEGF expression.Citation30 Recently, 15 different polymorphisms containing 460 C/T, 405 G/C, and 141 A/C in VEGF gene were determined. The most seen haplotypes in normal population are 460C/405G and 460T/405C. The VEGF 405 production is highest in GG genotype, is moderate in GC genotype, and finally is lowest in CC genotype has been shown in in vitro and in vivo studies.Citation3,Citation31 And also, the combination of genotype 405 and the other polymorphisms result in much more VEGF promotor activity.Citation32 A deletion/insertion polymorphism in VEGF promotor region at 2549 has been found related with an enhanced transcriptional activity.Citation33
Renner et al.Citation10 have found an association between VEGF plasma levels and VEGF 936 C/T gene polymorphism, and stated that patients with 936 T allele have significantly lower VEGF plasma levels.Citation34 Shahbazi et al.Citation11 have found that patients with coronary heart disease, who have VEGF 1154 G allele, have higher VEGF plasma levels. Chow et al.Citation30 have performed a study on patients with IgA nephropathy [119 female (61%) and 76 male (39%)]. They stated in their study that VEGF 2578 promoter CC genotype is associated with an increase in renal insufficiency.
In the literature, we could not encounter with a study investigating the relationship between VEGF C-936T and VEGF G-1154A polymorphisms and the remission rates in patients with glomerulonephritis. So, we aimed to investigate this relationship in our prospective study. However, we found no statistical significance between therapeutic outcomes and VEGF C-936T and VEGF G-1154A polymorphisms.
The results of this study are subjected to some limitations. First, this is a single-center study with a relatively small sample size. Second, we could not study serum VEGF levels and renal mRNA expression due to absence of commercial kits. However, it must be stated that measuring serum VEGF levels are not reliable in patients withglomerulonephritis under angiotensin converting enzyme inhibitor and corticosteroid therapy. In this setting, we suggest that serum VEGF levels might be measured before treatment.
In conclusion, the literature data suggest that VEGF and VEGF polymorphisms may be a marker in determining the progression in glomerular diseases.Citation29,Citation30,Citation35,Citation36 Although we could not demonstrate a statistical significance between therapeutic outcomes and VEGF C-936T and VEGF G-1154A polymorphisms, nonetheless more specifically designed prospective and observational studies in a larger cohort are needed to make certain conviction.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor in renal pathophysiology. Kidney Int. 2004;65:2003–2017
- Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Hematol. 2001;106(4):148–156
- Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE. Identification of polymorphism within the vascular endothelial growth factor gene: correlation with variation in VEGF protein production. Cytokine. 2000;12(8):1232–1235
- Iruela Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Hemost. 1997;78:672–677
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309
- Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954
- Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716
- Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18(11):2885–2893
- Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88(2):451–487
- Renner W, Kotschan S, Hoffman C, Obermayer-Pietsch B, Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res. 2000;37:443–448
- Shahbazi M, Fryer AA, Pravica V, Brogan IJ, Ramsay HM, Hutchinson IV. Polymorphisms in vascular endothelial growth factor gene are associated with increased risk of acute rejection in renal transplant recipients. J Am Soc Nephrol. 2002;13:260–264
- Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):560–576
- Gröne HJ, Simon M, Gröne EF. Expression of vascular endothelial growth factor in renal vascular disease and renal allografts. J Pathol. 1995;177:259–267
- Simon M, Gröne HJ, Jöhren O, et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240–F250
- Thomas S, Vanuysstel J, Gruden G, et al. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J Am Soc Nephrol. 2000;11:1236–1243
- Simon M, Röckl W, Hornig C, et al. Receptors of vascular endothelial growth factor/vascular permeability factor in fetal and adult human kidney: localization and [125I] VEGF binding sites. J Am Soc Nephrol. 1998;9:1032–1044
- Hakroush S, Moeller MJ, Theilig F, et al. Effects of increased renal tubular vascular endothelial growth factor on fibrosis, cyst formation, and glomerular disease. Am J Pathol. 2009;175:1883–1889
- Rudnicki M, Perco P, Enrich J, et al. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab Invest. 2009;89:337–346
- Ostendorf T, Kunter U, Eitner F, et al. VEBF(165) mediates glomerular endothelial repair. J Clin Invest. 1999;104:913–923
- Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC. VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer. 2010;46:439–448
- Khakoo AY, Sidman RL, Pasqualini R, Arap W. Does the rennin-angiotensin system participate in regulation of human vasculogenesis and angiogenesis? Cancer Res. 2008;68(22):9112–9115
- Ayerder EF, Haksun E, Ulver DB, et al. The relationship between vascular endothelial growth factor and microalbuminuria in patients with essential hypertension. Intern Med. 2008;47:1511–1516
- Honkanen EO, Teppo AM, Grönhagen-Riska C. Decreased urinary excretion of vascular endothelial growth factor in idiopathic membranous glomerulonephritis. Kidney Int. 2000;57:2343–2349
- Webb NJ, Watson CJ, Roberts IS, et al. Circulating vascular endothelial growth factor is not increased during relapses of steroid sensitive nephrotic syndrome. Kidney Int. 1999;55:1063–1071
- Cheong HI, Lee JH, Hahn H, et al. Circulating VEBF and TGFbeta1 in children with idiopathic nephrotic syndrome. J Nephrol. 2001;14:263–269
- Nitta K, Uchida K, Honda K, et al. Serum vascular endothelial growth factor concentration in rapidly progressive glomerulonephritis. Nephron. 1998;80:357–358
- Nitta K, Uchida K, Kimata N, et al. Increased serum levels of vascular endothelial growth factor in human crescentic glomerulonephritis. Clin Nephrol. 1999;52:76–82
- Matsumoto K, Kanmatsuse K. Elevated vascular endothelial growth factor levels in the urine of patients with minimal-change nephrotic syndrome. Clin Nephrol. 2001;55:269–274
- Ohashi R, Shimizu A, Masuda Y, et al. Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol. 2002;13(7):1795–1805
- Chow KM, Szeto CC, Lai FM, Poon P, Wong TY, Li PK. Genetic polymorphism of vascular endothelial growth factor: impact on progression of IgA nephropathy. Ren Fail. 2006;28:15–20
- Awata T, Inoue K, Kurihara S, et al. A common polymorphism in the 5 untranslated region of the VEBF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes. 2002;51:1635–1639
- Stevens A, Soden J, Brenchley PE, Ralph S, Ray DW. Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res. 2003;63:812–816
- Yang B, Cross DF, Ollerenshaw M, Millward BA, Demaine AG. Polymorphisms of the vascular endothelial growth factor and susceptibility to diabetic microvascular complications in patients with type 1 diabetes mellitus. J Diabetes Complications. 2003;17:1–6
- Biselli PM, Guerzoni AR, de Godoy MF, Pavarino-Bertelli EC, Goloni-Bertollo EM. Vascular endothelial growth factor genetic variability and coronary artery disease in Brazilian population. Heart Vessels. 2008;23:371–375
- Zeng HS, Xiong XY, Chen YY, Luo XP. Gene polymorphism of vascular endothelial growth factor in children with Henoch-Schonlein Purpura Nephritis. Chin J Contemporary Pediatr. 2009;11(6):417–421
- Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–1457