Abstract
Background: Calcific uremic arteriolopathy (CUA), previously called calciphylaxis, is a devastating complication of end-stage kidney disease (ESKD) with an annual incidence of 1–4% in dialysis patients and the mortality is as high as 80%. The rarity of the disease and the multifactorial nature of its causes have compromised good evidences that could determine the best therapy for the condition. For inhabitants in high-altitude area, the content of oxygen in the air is significantly lower than that in sea level area, which leads to the differences in the clinical manifestations and treatments to CUA. Case presentation: We presented a patient with CUA on Tibetan Plateau successfully treated by hyperbaric oxygen (HBO). This 46-year-old uremic Tibetan peasant received hemodialysis for 10 years, and over the last six months, skin necrosis occurred progressively on the distal joint of the middle finger of his right hand and the distal knuckles became paled, hardened, and severely painful. Extensive calcification of the arteries of both hands was revealed and his serum phosphorus elevated and serum calcium decreased. After diagnosis of CUA, patient was treated with HBO therapy for successive three weeks with a session per day, on the basis of secondary infection prevention. Pain of the affected finger was quickly alleviated in one week and the lesions of the affected finger healed in two months. Conclusion: As the dialysis population in high-altitude area increasing rapidly in recent years, this management strategy of improving focal oxygen supply by HBO might act as a reference for the treatment of CUA patients in similar conditions.
Background
Calcific uremic arteriolopathy (CUA), previously called calciphylaxis, is a devastating complication of end-stage kidney disease (ESKD), characterized by the deposition of calcium-phosphate products in small-vessel media leading to subsequent stenosis and thrombosis, and ultimately cause ulcers and dry gangrene of the soft tissues.Citation1 CUA has an annual incidence of 1–4% in dialysis patientsCitation1,Citation2 with mortality as high as 80%Citation2,Citation3 due to the complication of infection and sepsis. The rarity of the disease and the multifactorial nature of its causes have compromised good evidences that could determine the best therapy for the condition. For inhabitants in high-altitude area, the content of oxygen in the air is significantly lower than that in sea level area, which leads to the differences in the clinical manifestations and treatments to CUA.
We present here a patient with CUA on Tibetan Plateau successfully treated by hyperbaric oxygen (HBO). To our best knowledge, this is the first reported case of CUA treated in high-altitude area.
Case presentation
This 46-year-old male was a Tibetan peasant with long-term residence in areas 3700 m above the sea level who developed renal failure due to eight years of chronic glomerulonephritis and underwent hemodialysis for 10 years (5 h/session, 2 session/week). Right forearm autologous arteriovenous fistula (cephalic vein-radial artery anastomosis) was used as vascular access and functioned well. His mean blood pressure after dialysis was 140/90 mmHg.
Over the last six months, skin necrosis and ulcers occurred progressively on the distal joint of the middle finger of his right hand, the distal knuckles became paled, hardened and severely painful, followed by the distal ring finger of his left hand (). Radiologic examinations revealed bilateral osteoporosis of the hand and extensive calcification of the radial arteries, the ulnar arteries, the arterial arch of the palm, and arteries of the fingertips (). Calcification was suspected in the hilum as well. Color Doppler of the upper limbs showed massive atherosclerotic plaque within the cavity of the radial arteries and the ulnar arteries, and extensive calcification within the artery wall.
Figure 1. The finger affected by CUA in the uremic patient on Tibetan Plateau before and after treatment. (A) The appearance of affected finger: Focal ulcer on distal interphalangeal joint of the middle finger in his right hand, with pale and hardened distal finger. (B) X-ray image of the right hand showed extensive arteries calcification of arteries of the fingertips (indicated by white arrows). (C) Two months after treatment, the finger ulcer healed, with only tiny crust.
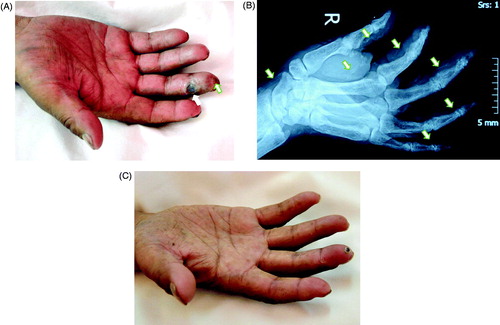
Blood tests showed elevated levels of serum phosphorus (3.55 mmol/L), iPTH (431 pg/mL) and hs-CRP (29.5 mg/L), and slightly decreased serum calcium (2.1 mmol/L). The hemoglobin level was 109 g/L, white blood cell count 8.5 × 109/L, fasting serum glucose 5.1 mmol/L. His lipid profile remained normal. The pulse oxygen saturation was 68–73% tested on his affected fingers and 93–95% on his other normal fingers.
CUA was diagnosed in this patient and mupirocin ointment was administered on the finger ulcers locally to prevent secondary infection. The patient was educated to avoid high-phosphorus diet intake and oral calcium-containing phosphate binding agents (calcium carbonate tablets) were given to reduce the absorption of phosphorus. Patient was treated with HBO therapy for successive three weeks with a session per day (23% oxygen, 0.25 MPa, 90 min per session).
Pain of the affected finger was alleviated in one week after HBO therapy start. Upon the one-month follow-up, ulcers of finger significantly narrowed and the stiff knuckle started to turn red and soft. Blood tests demonstrated a phosphorus level of 1.4 mmol/L, a serum calcium level of 2.21 mmol/L. The iPTH level was 330 pg/mL and hs-CRP level was 15 mg/L. Two months later, the affected finger completely healed, leaving only needle cap-sized crusts covering the fingertips (). Skin temperature and color had returned to normal as well as the motor function of the fingers. The pulse oxygen saturation of the affected finger was 92–93%.
Conclusions
CUA, as a late complication of ESKD, is rare and the typical histological change of CUA is small vessel calcification, triggering the intimal proliferation, endovascular fibrosis, and thrombosis. By now, no therapeutic recommendations based on randomized controlled trials are available for this disorder. Misdiagnosis and inappropriate managements might further delay the treatment. Prognosis is usually disappointing once proximal CUA affecting the abdomen, the thighs and buttocks occurs, thereby resulting in a mortality rate of 63%.Citation4,Citation5 Distal CUA is associated with a lower mortality rate, reported as 23% in one study.Citation5–7
Various managements, such as medical therapy (sodium thiosulfate, unfractionated heparin, cinacalcet, and corticosteroids), surgery (parathyroidectomy), low calcium, or calcium-free dialysate for hemodialysis and focal management of the wound (HBO therapy, surgical debridement of devitalized tissue) were reported for this rare and thorny condition.Citation8–11 However, a systematic strategy remains controversial.
For general ESKD patients in low-altitude area, HBO therapy showed efficacy in some reports. Vassa et al.Citation12 have reported one case of CUA managed by HBO therapy and satisfactory outcomes were achieved. Rogers et al.Citation13 found the beneficial effect of adding HBO treatment in promoting wound healing and thus reducing mortality associated with CUA. In a systematic review published in 2011,Citation11 46 CUA cases in low-altitude area treated with HBO were published, with 34 noting success. However, for CUA patients in high-altitude area, study data is limited.
The patient presented in this case report is a long-term Tibetan resident living at an altitude of over 3700 m above the sea level, where the oxygen content in the air is about 60% of that at the sea level. The low oxygen content associated with the high altitude is believed to be one of the important precipitating factors of vascular ischemia and calcification as well as further tissue damage. During the management of this patient, considerable attentions were given to focal oxygen supply improvement, in addition to baseline treatment such as controlling phosphate level and local infection prevention. This strategy worked well for this case.
As the number of patients receiving long-term dialysis at high-altitude environment keep increasing in recent years (like that in Tibet), this management strategy of improving focal oxygen supply by HBO on the basis of good serum phosphorus control and infection prevention might act as a reference for the treatment of patients with similar conditions.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Zhiguo Mao is an Outstanding Young Scholar of Second Military Medical University. This work was supported by Chinese Society of Nephrology (No. 13030340419), Major Fundamental Research Program of Shanghai Committee of Science and Technology (No. 12DJ1400300) and Key Projects in the National Science & Technology Pillar Program in the Twelfth Five-year Plan Period (No. 2011BAI10B00).
Authors’ contributions
GX, YD and CL treated the patients and collected the data, HZ, BY and XC wrote the manuscript. ZM and CM designed the study and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61(6):2210–2217
- Angelis M, Wong LL, Myers SA, Wong LM. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122(6):1083–1089; discussion 1089–1090
- Rogers NM, Coates PT. Calcific uremic arteriolopathy: an update. Curr Opin Nephrol Hypertens. 2008;17(6):629–634
- Bleyer AJ, Choi M, Igwemezie B, de la Torre E, White WL. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32(3):376–383
- Mohammed IA, Sekar V, Bubtana AJ, Mitra S, Hutchison AJ. Proximal calciphylaxis treated with calcimimetic ‘Cinacalcet’. Nephrol Dialysis Transplant. 2008;23(1):387–389
- Arseculeratne G, Evans AT, Morley SM. Calciphylaxis—a topical overview. J Eur Acad Dermatol Venereol. 2006;20(5):493–502
- Hussein MR, Ali HO, Abdulwahed SR, Argoby Y, Tobeigei FH. Calciphylaxis cutis: a case report and review of literature. Exp Mol Pathol. 2009;86(2):134–135
- Chan YH, Wong KM, Kwok PH, et al. Atypical presentation of calciphylaxis in a patient with renal failure: successful treatment with unfractionated heparin. HongKong J Nephrol. 2001;3(2):103–106
- Llach F. The evolving pattern of calciphylaxis: therapeutic considerations. Nephrol Dialysis Transplant. 2001;16(3):448–451
- Llach F, Bover J. Renal osteodystrophy. In: Brenner BM, ed. The Kidney. 6th ed. Philadelphia: WB Saunders Company; 2001:2128–2130
- Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol. 2012;67(6):e253–e260
- Vassa N, Twardowski ZJ, Campbell J. Hyperbaric oxygen therapy in calciphylaxis-induced skin necrosis in a peritoneal dialysis patient. Am J Kidney Dis. 1994;23(6):878–881
- Rogers N, Chang S, Teubner D, Coates P. Hyperbaric oxygen as effective adjuvant therapy in the treatment of distal calcific uraemic arteriolopathy. Nephrol Dial Transplant Plus. 2008;1:244–249
