Abstract
Background: Some children with idiopathic nephrotic syndrome (NS) patients fail to respond even when given high dose of steroid. The aim of this study was to assess the GCR expression on the T lymphocytes of children with NS and its relation to the response to steroid and to histopathological type. Methods: Forty-six pediatric patients with idiopathic NS and 20 age and sex matched apparently healthy children as controls were included. Flow cytometry was employed to determine the percentage of CD3+/GCR+ cells which then correlated with pattern of steroid response. Renal biopsy was done for steroid-dependent and steroid-resistant cases for determination of the underlying histopathological type. Results: The mean percentage of T lymphocyte expression of GCRs (CD3+/GCR) was significantly higher in early steroid responders than in late responders and was slightly lower than the controls. There was a significantly lower GCRs expression in steroid-resistant patients in comparison to early responders, late responders and controls. Renal biopsy showed that most cases of late responders were of the focal segmental glomerulosclerosis (FSGS) type. The mean percentage of lymphocyte expression of GCRs was significantly higher in patients with minimal change disease (MCD) compared to patients with FSGS. Conclusion: Evaluation of the expression of intracellular GCRs in T lymphocytes at time of diagnosis of NS can predict the response to steroid therapy and can help in determination of the outcome of NS patients regarding future relapses.
Introduction
Nephrotic syndrome (NS) is one of the most frequently seen kidney diseases in children characterized by massive proteinuria, hypo-albuminemia and edema.Citation1 NS is a clinical manifestation of different histopathological subtypes based on histopathological finding on renal biopsy.Citation2
Glucocorticoids (GCs) are among the most effective drugs for the treatment of NS. However, not all children, who initially appear to have similar histological and clinical features of NS, respond to steroid therapy.Citation3
The response to GCs treatment is an important indicator for the outcome of the idiopathic NS in children. A good initial response of proteinuria to GCs is usually associated with an eventual cure, even after multiple inter-current relapses. In contrast, an incomplete or missing response may herald the progression to renal failure.Citation4 Renal biopsy in these steroid-resistant patients provides important information on renal histology and outcome.Citation5 Within the general population there is substantial, yet stable, inter-individual variability in the clinical responsiveness to endogenous and therapeutic GCs. Although decreased responsiveness can have multiple origins, it is often caused by structural or functional abnormalities in the glucocorticoid receptors (GCRs). Such alterations can affect GCR ligand affinity, receptor number or the ability to mount an appropriate transcriptional response.Citation6 Evaluation of GCR expression in children by a suitable method before treatment might be beneficial in predicting response to steroid. Prediction of clinical response before starting steroid therapy may lead to administration of synergized therapy at the beginning of the treatment and this will help to avoid the side effects of chronic ineffective high-dose hormone therapy, improve the individual response to GC therapy and benefit more patients.Citation7
The aim of this study was to assess the GCR expression in the T lymphocytes of children with NS and its relation to the response to GCs and to the histopathological type.
Patients and methods
This study was a prospective case–controlled study included 46 pediatric patients with idiopathic NS. The patients were recruited from the Pediatric Nephrology Unit of the Pediatric Department, Assiut University Children Hospital, from January 2012 to 2013. Twenty age- and sex- matched apparently healthy children were recruited as a control group. The study was approved by the Institutional Review Board of the Faculty of Medicine, Assiut University. An informed written consent in accordance with Assiut University Ethical Committee guidelines was taken from guardians of all cases and controls.
At diagnosis, all patients were subjected to complete history taking, thorough clinical examination, measurement of 24-h urinary protein, serum albumin, serum urea and creatinine (Cobas Integra 400 Chemistry Analyzer, Roche Diagnostics GmbH, Mannheim, Germany), complete blood count (Celltac E automated hematology analyzer, Tokyo, Japan). The expression of intracellular GCRs was measured by flow cytometry.
NS was defined as edema, proteinuria greater than 40 mg/m2/h, and hypo-albuminemia. At the time of the initial diagnosis, prednisone, 60 mg/m2/d, was started. Remission was defined as urinary protein excretion (proteinuria) < 4 mg/h/m2 or Albustix negative or trace for 3 consecutive days.Citation8 Based on the response to steroid treatment, the patients were divided into three groups; early responders in whom complete remission was achieved within 4 weeks of steroid treatment, late responders in whom complete remission was achieved after 4 weeks of steroid treatment, and steroid-resistant group who fail to respond to steroid after 8 weeks of treatment.Citation9
All the steroid sensitive patients were followed-up during and after gradual withdrawal of steroid for up to 6 months to detect relapsing and steroid-dependent cases. Relapse was diagnosed when proteinuria occurred again (> 40 mg/m2/h) for 3 consecutive days during or after steroid withdrawal. Steroid dependant cases was defined when relapse occurs during the corticosteroid withdrawal or within 28 d of completing a successful course of corticosteroid therapy.Citation8
Histopathology
Renal biopsy was done for steroid-dependant and steroid-resistant cases for determination of the underlying histopathological type. Renal biopsy was done using 10-cm long, 18-Gauge Spring loaded True cut biopsy needle with automated gun after obtaining appropriate informed consent. Two cores of native renal biopsy were routinely obtained under ultrasound guidance for evaluation by light microscopy. Biopsy specimens were processed using standard procedures. Six sections were done and specimens were stained with hematoxylin–eosin, periodic acid-Schiff, and Reticulin. The biopsy specimens were reviewed and interpreted by the same pathologists. Adequacy of biopsy was defined as the presence of at least five glomeruli in the specimen on light microscopy. Minimal change disease (MCD) was characterized by the absence of any conspicuous abnormality on light microscopy. Focal segmental glomerulosclerosis (FSGS) was characterized by the presence of at least one glomerulus showing a segmental area of sclerosis with or without accompanying tubular atrophy and interstitial fibrosis.Citation10
Flow cytometric detection of glucocorticoid receptors
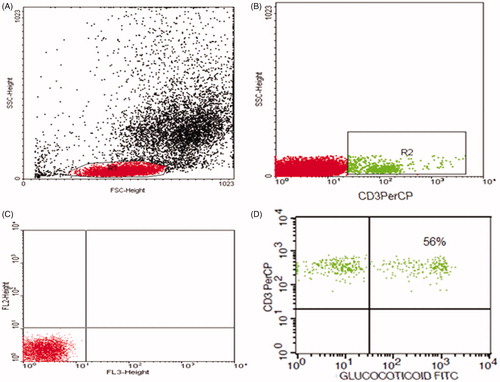
Pyridinium-chlorophyll-protein (Per-CP)-conjugated antihuman CD3 and was used to detect T lymphocytes. Mouse anti-human anti-GCR monoclonal antibody and anti-mouse IgG-FITC were used for the detection of GCRs. All monoclonal antibodies were purchased from Becton Dickinson Biosciences, San Jose, CA. A total of 50 μL of blood sample was incubated with 5 μL of anti-CD3 for 20 min. Following incubation, RBC lysis, washing with phosphate buffer saline (PBS), addition of fixative solution to fix the cells and incubation for 10 min were done. Then the cells were washed with PBS, and permeabilization solution and anti-GCR were added and incubated for 60 min. After washing, 5 μL of anti-mouse IgG-FITC was added and incubation for 30 min was done. After extensive washing with PBS to remove unbound secondary antibodies, flow cytometric analysis was done by FACSCalibur flow cytometry with CellQuest software (Becton Dickinson Biosciences, San Jose, CA). FITC-labelled IgG was used as an isotype-matched negative control with each sample. At least 20,000 events in the light-scatter (SSC/FSC) were acquired. A CD3+ population was gated and its expression of GCRs was detected ().
Figure 1. Flow cytometric detection of glucocorticoid receptor in T lymphocytes (CD3+/GCRs). (A) Forward and side scatter histogram was used to define the lymphocytes population (R1). (B) CD3+ cells was gated within the lymphocytes population (R1), compared with the negative isotype control (C). (D) The expression of glucocorticoid receptors within CD3+ cells was detected.
Statistical analysis
Data analysis was done by statistical package for social sciences (SPSS, Chicago, IL), version 16. All data were expressed as the mean ± standard deviation of mean (SD). Differences between the groups were examined for statistical significance using Independent Sample t test and one-way analysis of variance. A p value of ≤ 0.05 denoted the presence of a statistically significant difference. Pearson correlation coefficient was used to examine the correlations among different studied parameters.
Results
Demographic characteristics and some laboratory findings of the study population were shown in . Based on the response to GCs treatment, there were 15 early steroid-responder patients, 17 late steroid-responder patients and 14 steroid-resistant patients. Steroid dependence was detected in 15 patients (11 were originally late steroid responders and 4 were early steroid responders).
Table 1. Baseline characteristics of nephrotic syndrome patients and the controls.
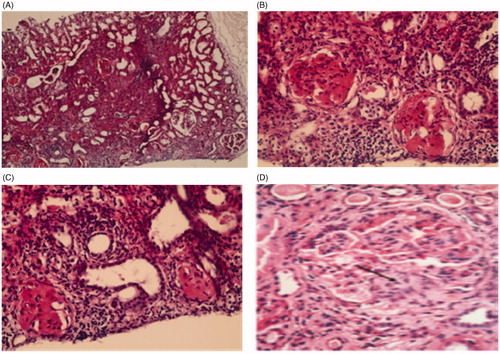
Renal biopsy was done in 29 patients (14 steroid resistant and 15 steroid dependants). Sixteen patients had MCD (12 patients were steroid dependant and 4 patients were steroid resistant) and 13 patients had FSGS disease (3 patients were late steroid responders and 10 patients were steroid resistant; ).
Figure 2. Histopathological pattern of nephrotic syndrome. (A) Section showed focal affection of glomeruli, tubular atrophy and interstitial fibrosis H & E × 100. (B) Glomeruli showed segmental area of sclerosis with mesangial proliferation, and interstitial fibrosis H & E × 400. (C) Glomeruli showed segmental area of sclerosis, tubules atrophied with interstitial fibrosis H & E × 400. (D) Section showed lobulated glomerulus with segmental glomerulosclerosis (arrow) and moderateglobal hypercellularity, H & E × 400.
The mean percentage of T lymphocyte expression of GCRs (CD3+/GCR) in early steroid responders was significantly higher than in late responders and it was slightly lower than the controls but with no significant difference. The mean percentage of expression of GCRs was significantly lower in the late responder than that in the controls. There was a significantly lower GCRs expression in steroid-resistant patients in comparison to all early responders, late responders and the controls ().
Table 2. Glucocorticoid receptors expression in newly diagnosed nephrotic syndrome.
Comparing patients according to the results of renal biopsy, the mean percentage of lymphocyte expression of GCRs was significantly higher in patients with renal biopsy with MCD (66.82 ± 12.44) compared to the patients with FSGS (57.03 ± 15.48), p = 0.026.
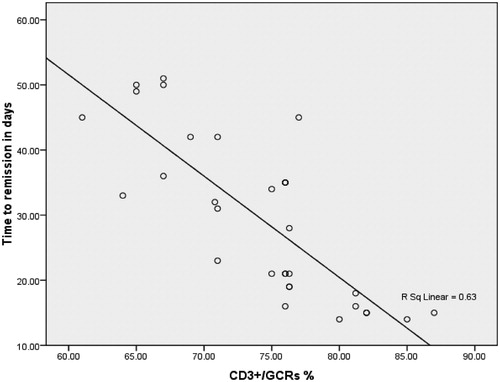
The time interval from the start of steroid therapy to achievement of complete remission was significantly longer in the late steroid responders (34.77 ± 11.31 d) than in the early steroid responders (17.6 ± 3.27 d), p = 0.009. There was a negative correlation between the expression of GCRs in T lymphocytes (CD3+/GCRs) and time interval from starting steroid therapy to time achieving complete remission, r = −0.802 ().
Discussion
One of the most important indicators for the outcome of children with idiopathic NS is their initial response to steroid therapy. To date, no satisfactory explanation has been provided as to why some NS patients respond to steroid and others do not.Citation11 Cellular sensitivity to GC is influenced by the level and structure of GC receptors, their affinity for ligand, their rate of translocation to the nucleus and their ability to transactivate the response.Citation12
In the present study, there was a significance lower GCR expression in steroid-resistant patients in comparison to early steroid responders, late steroid responders and control. Evaluation of GCR expression in children might be valuable in predicting response to steroid. Bagdasorova et al.Citation13 found an increase in GCR expression in patients with steroid sensitive and decrease in steroid resistance NS. Similar results were obtained by Ayman et al.,Citation14 who found lower GCR expression on lymphocytes in steroid resistance NS in comparisons to steroid sensitive NS and control. Han et al.Citation15 showed that glomerular GCR expression was significantly lower in late than in early steroid responder adults with NS. On the other hand, the current study is at variance with Haack et al.Citation16 who determined GCR in different types of glomerulonephritis and did not find any difference in density and binding affinity of GCR in mononuclear leukocytes between steroid-sensitive and steroid-resistant NS children. Also, the present study is in variance with Peisong et al.Citation17 who found no significant difference of the total GCR expression between glucocorticoid-sensitive and glucocorticoid-resistant patients at both mRNA and protein levels. The differences between our results and the previous studies could be explained by the different methods used in assessment of GCR in these studies.
The significant reverse correlation between the expression of GCR and the time interval from the start of steroid treatment to remission in our steroid responder patients could add value to importance of evaluation of GCR expression in children with NS. Our result is in accordance with Shalaby et al.Citation18 and Han et al.,Citation19 who found significant inverse correlation between the expression of GCR and time interval from the start of steroid therapy to complete remission in patients with NS.
Follow-up of our cases revealed that most of late responder patients, who had lower GCR expression, had relapsing or steroid-dependent course of the disease. In contrast, most of the early responder patients, who had higher GCR expression, showed non-relapsing course during period of follow-up. This is in accordance with Vivarelli et al.Citation20 who concluded that the length of time between steroid treatment onset and remission is a prognostic indicator for patients with idiopathic NS.
So, the pattern of steroid response either dependent or resistant may be dependent on the level of expression of GCR on peripheral lymphocytes, this pattern can be expected early in the course of treatment by estimation of level of GCR. However, estimation of cut-off points of different patterns from larger study number will be very helpful in this field. The early identification of children at high risk of steroid resistance or dependence requiring steroid sparing agents may be a useful diagnostic tool to tailor the therapeutic strategy.Citation21
MCD is the most common pathological finding of NS syndrome in children accounting of more than 75% of cases. Most of who promptly respond to glucocorticoid.Citation15 Renal biopsy was done in 29 patients (14 steroid resistant and 15 steroid dependent). Sixteen patients had MCD (12 patients were steroid dependant and 4 patients were steroid resistant) and 13 patients had focal segmental disease (3 patients were late steroid responder and 10 patients were steroid resistant). In the present study, the majority of cases in late responders were FSGS. This is in accordance with Gulati et al.Citation22 from India who reported FSGS in 59% of steroid-resistant cases. Also, in Saudi Arabia and Tunisie (countries comparable to Egypt), the incidence of FSGS is more important than MCD in steroid-resistant cases.Citation23,Citation24 However, two studies from Japan and France reported a significantly higher incidence of MCD compared to FSGS in steroid-resistant NS.Citation11,Citation24 The reasons for disparities in the frequency of MCD and FSGS are not entirely clear. They are probably explained by racial, genetic and environmental factors. Several studies documented that some patients with late responders whose initial kidney biopsy specimens was MCD, subsequent follow-up biopsy showed FSGS change. This has led to the conclusion that MCD and FSGS may represent a continuum of the same process.
In the present study, the mean percentage of lymphocyte expression of GCRs was significantly higher in patients with MCD renal biopsy compared to patients with FSGS who represent majority of cases of late responders. So we suspect that, in patients with low GCR expression and low immune modulation MCD disease may progress to FSGS. Further studies on large scale are needed in children with NS of different sensitivity to GCs to confirm this hypothesis.
Conclusion
Evaluation of the expression of intracellular GCRs in T lymphocytes at the time of diagnosis of NS can predict the response to steroid therapy and can help in determination of the outcome of NS patients regarding future relapses. Most cases of late responders were of the FSGS type and most of them have low GCR expression.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Feliciau E. Pediatric nephrology around the world – Africa. In: Ellis D, William AE, eds. Text Book of Pediatric Nephrology. 6th ed., Vol. 82. New York, NY: Harmon and Patrick Niaduet; 2009;1962–1968
- Gulati S, Sharma AP, Sharma RK, Gupta A. Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis. 1999;34(4):646–650
- Wasilewska A, Zoch-Zwierz W, Tomaszewska B, Wierciński R, Stasiak-Barmuta A. Expression of glucocorticoid receptors in mononuclear cells in NS. Pediatr Nephrol. 2003;18(8):778–782
- -Chen T, Lv Y, Lin F, Zhu J. Acute kidney injury in adult idiopathic NS. Renal Failure. 2011;33(2):144–149
- Gargah T, Labassi A, Goucha-Louzir R, Ben Moussa F, Lakhoua MR. Histopathological spectrum of childhood idiopathic steroid-resistant nephrotic syndrome in Tunisia. Tunis Med. 2011:89(3):258–261
- Jorge A Iniguez-Lluhi. Glucocorticoid receptor function in steroid resistant childhood nephrotic syndrome. National institute of diabetes and digestive and kidney diseases syndrome in Egyptian children. J Nephrol. 2011:25(5):732–737
- Habashy D, Hodson E, Craig J. Interventions for idiopathic steroid-resistant NS in children. Cochrane Database Syst Rev. 2004:(2):CD003594
- Pais P, Ellis D. Nephrotic Syndrome, Nelson Textbook of Pediatrics, 19th ed., Chapter 5. Philadelphia (PA): Saunders; 2012:1801–1807
- Gulati S, Sengupta D, Sharma RK, et al. Steroid resistant nephrotic syndrome: role of histopathology. Indian Pediatr. 2006:43:55–60
- Mohammad N, Khan TM, Orakzai AN, Imran M. Histological pattern of glomerulopathies. Gomal J Med Sci. 2012:10(1):7(1–11)
- Mekahli D, Liutkus A, Ranchin B, et al. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr Nephrol. 2009:24(8):1525–1532
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996:17:245–261
- Bagdasarova IV, Ivanov DD, Afanas’eva VV. The morphofunctional characteristics of the blood lymphocytes in steroid sensitive and steroid-resistant glomerulonephritis. Arkh Patol. 1991:53:28–32
- Hammad A, Yahia S, Gouida MS, Bakr A, El-farahaty RM. low expression of glucocorticoid receptors in children with steroid resistance nephrotic syndrome. Pediatr Nephrol. 2013:28(5):759–763
- Han SH, Park SY, Li JJ, et al. Glomerular glucocorticoid receptor expression is reduced in late responders to steroids in adult-onset minimal change disease. Nephrol Dial Transplant. 2008:23(1):169–175
- Haack D, Scharer K, Asam-Tauscher A, Vecsei P. Glucocorticoid receptors in idiopathic nephrotic syndrome. Pediatr Nephrol. 1999:13:653–656
- Peisong C, Tang J, Ouyang J, Yingpeng C. Glucocorticoid receptors auto regulation and its relation with glucococrticoid sensitivity in idiopathic nephritic syndrome. Int Urol Nephrol. 2011:43:167–174
- Shalaby SA, El Idrissy HM, Safar RA, Hussein ST. Glucocorticoid receptors and the pattern of steroid response in idiopathic NS. Arab J Nephrol Transplant. 2012:5(1):13–17
- Han SH, Park SY, Li JJ, et al. Glomerular glucocorticoid receptor expression is reduced in late responders to steroids in adult-onset minimal change disease. Nephrol Dial Transplant. 2008:23(1):169–175
- Vivarelli M, Moscaritolo E, Tsalkidis A, Massella L, Emma F. Time for initial response to steroids is a major prognostic factor in idiopathic NS. J Pediatr. 2010:156(6):965–971
- Harambat J, Godron A, Ernould S, Rigothier C, Llanas B, Leroy S. Prediction of steroid-sparing agent use in childhood idiopathic nephrotic syndrome. Pediatr Nephrol. 2013:28(4):631–638
- Gulati A, Bagga A, Gulati S, Mehta KP, Vijayakumar M. Management of steroid resistant nephrotic syndrome. Indian Pediatr. 2009:46(1):35–47
- Kari JA, Halawani M, Mokhtar G, Jalalah SM, Anshasi W. Histopathology of steroid-resistant nephrotic syndrome in children living in the Kingdom of Saudi Arabia. Pediatr Nephrol. 2009:24:1429–1430
- Hamasaki Y, Yoshikawa N, Hattori S, et al. Japanese Study Group of Renal Disease. Cyclosporine and steroid therapy in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2009:24(11):2177–2185